The paper is open access. To read the original paper click here, A Spanish Dancer…
You can also find it in the Bird’s Head Seascape’s website Library.
Abstract
Color ontogeny and variations associated with discrete morphological differences may generate taxonomical challenges, which requires multiple data types and in-depth historical review. The nudibranch known as the Spanish dancer, Hexabranchus sanguineus, is a classic example with over 200 years of taxonomic confusion. Currently, H. sanguineus is accepted by most authors as a single species from the Indo-Pacific Ocean with Hexabranchus morsomusas a valid species from the Atlantic Ocean. Yet, despite these species being highly studied, their systematic status remains debatable. Over 30 synonyms have been proposed for H. sanguineus and even a distinct genus for H. morsomus. Here we provide, for the first time, a comprehensive review of all proposed names and an integrative taxonomic revision of the genus including morphological and molecular data. Our results reveal that H. sanguineus is a complex of five species: four previously described and an undescribed species, one of the largest nudibranchs in the world: Hexabranchus giganteus sp. nov. The genus Caribranchusis considered a junior synonym of Hexabranchus Ehrenberg, 1828 and the ontogeny of color pattern is discussed.
Introduction
Hexabranchidae Bergh (1891) is a small family of large nudibranchs that melds plesiomorphic characters (e.g., simple hamate teeth) and derived characters (e.g., a differentiated prostate) (Marcus & Marcus, 1962; Ortea et al., 2002). Hexabranchidae differs from other dorid nudibranch families in bearing a circle of separate gill tufts around the anus instead of a single gill pocket (Eliot, 1904a). This family is currently considered by most authors to contain the single genus Hexabranchus Ehrenberg (1828), which, in turn, contains two species: Hexabranchus sanguineus (Rüppell & Leuckart, 1830) and Hexabranchus morsomus Marcus and Marcus (1962). In contrast, Ortea et al. (2002) disagreed that Hexabranchus is the only genus in the family due to the distribution and differences in the reproductive and digestive systems of H. sanguineus and H. morsomus. These authors proposed the genus CaribranchusOrtea et al. (2003) to accommodate H. morsomus. Nevertheless, with rare exceptions (Debelius & Kuiter, 2007; Gutiérrez et al., 2015; Ortea & Buske, 2018; Ortea et al., 2012), this name has been ignored in the literature. Valdés et al. (2006) regarded the genus Caribranchusas a synonym of Hexabranchus due to the lack of phylogenetic evidence. In MolluscaBase eds (2023a), this genus is cited as unaccepted, but no further discussion could be found.
How many Hexabranchus species are there? This question has already been the title of a manuscript (Valdés, 2002) and has been heavily debated in the literature (Bergh, 1878; Eliot, 1904a), yet it is poorly answered. Of all nudibranch species, the “Spanish dancer” (H. sanguineus) is one of the most famous and most puzzling with over fifty names attributed to it. Some have been forgotten, others poorly presented, and many synonymized. The genus Hexabranchus Ehrenberg (1828) was first erected to include two species: Hexabranchus praetextus Ehrenberg (1828) from Egypt and Doris lacera Cuvier, 1804 from Timor. Subsequently, Abraham (1876) transferred another eight species of Doris to this genus, including Doris sanguinea Rüppell & Leuckart, 1830 (from Egypt). Soon after, Bergh (1878) doubted that all these species were valid, suggesting that they were likely variations of the same species. Bergh (1900) presented a list of 17 species he believed were synonyms of H. lacer. Eliot (1904b, 1908) noted that Hexabranchus is highly variable in color, preserved specimens are often deformed, the shape of living animals changes with the animal’s movement (from oval to elongate) and the radula is not particularly informative. He added that even the same specimen can change colors, as animals in captivity were able to change their tonality within hours (Eliot, 1904b). Because of incongruencies in the description of H. lacer and confusions regarding to the year of publication of the description of H. praetextus(see details below), Eliot (1908) suggested that Hexabranchus sanguineus (Rüppell & Leuckart, 1830) should be the valid name for several morphotypes of Hexabranchus species. Despite this, new variations continued to appear and additional species were described (e.g., Bergh, 1905; Ostergaard, 1955). Marcus and Marcus (1962) also suspected that all Indo-Pacific species were the same, but described a new species (Hexabranchus morsomus Marcus & Marcus, 1962) from the Caribbean which was clearly differentiated by its geographic distribution and radula. This hypothesis was discussed by Thompson (1972) who explicitly synonymized 20 Indo-Pacific species as H. sanguineus and left the taxonomic status of H. morsomus as uncertain. Thompson (1972) did not provide a detailed comparison between the synonymized species but, since his publication, H. sanguineus has been accepted by most authors as the single Indo-Pacific species. Even through, many researchers doubted Thompson’s (op.cit.) conclusion (e.g., Edmunds, 1968; Francis, 1980; Yonow, 2001, 2008). In an attempt to resolve this question, Valdés (2002) provided a morphological review of the genus. In this review, he considered H. morsomus from the Atlantic Ocean to be a valid species but concluded that all species from the Indo-Pacific were the same including a further 15 names in the list of synonyms of H. sanguineus. As a result, the WoRMS database (MolluscaBase eds., 2023b) and most recent studies accept H. sanguineus as the single species in the Indo-Pacific. Yet, this hypothesis remains largely untested.
Yonow (2001, 2008) argued that H. sanguineus is consistently deep red and limited to the Red Sea. According to this author, other forms from the Indo-Pacific belong to H. marginatusand a larger pink form with a subpustulate notum is likely a different species. Pittman (2011) provided an extensive on-line review of several thousand photographs of Hexabranchus spp. and hypothesized that the genus contains at least three species in the Indo-Pacific and perhaps eight. In order to clear up over 200 years of taxonomic confusion and clarify the taxonomic status of Hexabranchus species, we provide a full review of all proposed names and investigate several morphotypes of this enigmatic complex applying integrative taxonomy.
Material and methods
Taxon sampling
Specimens and/or tissue samples were obtained directly by SCUBA diving or snorkeling and through loans from the California Academy of Science (CASIZ), the University Museum of Bergen (ZMBN), the Florida Museum of Natural History (UF), and the Museums Victoria (NMVF). Collected specimens were relaxed by freezing or in isotonic magnesium chloride solution and fixed in ethanol (70–96%). All material collected was deposited at the University Museum of Bergen (ZMBN), the Coleção de História Natural da Faculdade de Ciências da Universidade do Lúrio (UL), the Museu Nacional de História e da Ciência de Lisboa (MB), the Museo Nacional de Ciencias Naturales de Madrid (MNCN), and the Museu de História Natural de Maputo (MHN). Table 1 provides voucher numbers and summarizes the material utilized for molecular studies.
Photograph review
Hexabranchus species have a complex ontogeny of external body form and coloration. Unfortunately, not all morphotypes were available for this study. Therefore, to fill ontogenetic and distribution gaps, several thousand photographs from published guides and a variety of websites were examined and are discussed where pertinent. In particular, the following websites/database were used: iNaturalist (https://www.inaturalist.org), MedSlug (http://www.medslugs.de), South-west Indian Ocean Seaslug site (http://seaslugs.free.fr/nudibranche/a_intro.htm), NudiPixel (internet archive, https://web.archive.org/web/20121104103031/http://www.nudipixel.net), Sea Slug Forum (http://www.seaslugforum.net), Sea Slug of Hawai`i (http://seaslugsofhawaii.com), Sea Slug World (https://seaslug.world), Nudibranchs Sunshine Coast Queensland, Australia (https://nudibranch.com.au), and Underwater Australasia (https://underwater.com.au/image/id/6343-dance-with-me-/). All illustrations of Hexabranchus spp. ontogeny and the distribution map are marked to clearly differentiate between morphotypes that have been directly examined for this study and those known only from photographic material.
Anatomical work
Specimens were dissected by dorsal insertion. Small specimens (< 50 mm) were fully dissected under a stereo microscope while large individuals were first dissected by eye with small parts further examined under a stereo microscope. The reproductive system and buccal mass were separated. Drawings of internal organs were made with the aid of a camera lucida when size allowed. In the case of large specimens, drawings were made using a drawing table in Adobe Photoshop (v. 2021) by combining scaled photographs and direct examination. The jaws and radula were immersed in a solution of sodium hydroxide (NaOH) until clean. The radula and jaws were first examined under an optical microscope and then mounted for scanning electron microscopy (SEM).
DNA extraction, amplification, and sequencing
DNA was extracted from foot tissue samples using a Qiagen DNease Blood and Tissue kit (Qiagen, Valencia, CA, USA) following the manufacturer’s protocol. Attempts were made to obtain three genes: COI, 16S, and H3. The genes were amplified using the universal primers: LCO1490 (F) GGTCAACAAATCATAAAGATATTGG, HCO2198 (R) TAAACTTCAGGGTGACCAAAAATCA) (Folmer et al., 1994), 16S rRNA (primers: 16S ar-L (F) CGCCTGTTTATCAAAAACAT, 16S br-H (R) CCGGTCTGAACTCAGATCACGT) (Palumbi et al., 2002), and histone H3 (primers: H3AD5′3′: (F) ATGGCTCGTACCAAGCAGACVGC, H3BD5′3′ (R) ATAT- CCTTRGGCATRATRGTGAC) (Colgan et al., 1998) following the protocols detailed in Table 2. PCR products were analyzed using gel electrophoreses. Successful PCR products were purified at the University of Bergen using the EXO-SAP method with exonuclease 1 (EXO, 10 units µL−1) and shrimp alkaline phosphatase (SAP, 1 unit mL−1, USB) in 25-mL reactions (EXO 0.25 mL, SAP 2.5 mL, Sigma-Aldrich water 2.25 mL, and PCR product 20 mL) and run on a thermal cycler at 37 °C (incubation) for 30 min followed by 15 min at 80 °C (enzyme inactivation) or sent to Macrogen, Inc. (Madrid, Spain) for purification. All successful PCR products were sequenced by Macrogen, Inc.
Phylogenetic analysis and species delimitation
Sequences were examined, aligned, and concatenated in Geneious v.10.2.4 (Biomatters, Auckland, New Zealand) (Kearse et al., 2012). Multiple sequence alignments were obtained using MUSCLE with default settings, i.e., a maximum of eight interactions and grouping sequences by similarity and anchor optimization (Edgar, 2004). The absence of stop codons was verified for protein-coding genes (Genetic Code: Invertebrate) through the Geneious translation tool. Contamination was checked using BLAST implemented in GenBank (Altschul et al., 1990). The alignment of the mitochondrial 16S gene was reviewed in Gblocks Server 0.9 lb (Instituto de Biología Evolutiva CSIC-UPF, Barcelona, Spain) for hypervariable regions under less stringent settings (Castresana, 2000).
The best-fit evolutionary models of each gene were selected using the software jModelTest ver. 2.1.7 (Universidad de Vigo, Vigo, Spain) (Darriba et al., 2012) applying seven gene substitution schemes and generating 56 different models under the Akaike information criterion (Akaike, 1974). The best fit-model selected for the COI and 16S genes was GTR + I + G and for the H3 gene was GTR + G.
Bayesian phylogenetic analyses (BI) were carried out for the concatenated alignment (COI + 16 + H3) in MrBayes (Ronquist & Huelsenbeck, 2003) with four chains and two parallel runs of five million generations. The analysis was portioned by gene using the “unlink” command with 25% burn-in. A maximum likelihood (ML) analysis was carried out using MEGAX v. 10.2.4 applying default settings (Kimura 2-parameter substitution model, rates gamma G + I) with 1000 bootstrap replicates. maximum parsimony (MP) analyses were performed in PAUP* 4.0a167 by heuristic search under “tree bisection-reconstruction” branch swapping (TBR) and 1000 random replicates. Gaps were treated as missing data and all characters were unweighted. Node robustness was assessed using 1000 nonparametric bootstrap replicates. Nodes supported by BS ≥ 75 and PP ≥ 0.90, MP ≥ 70 were considered significant (Alfaro et al., 2003; Huelsenbeck & Rannala, 2004).
The trees generated by MrBayes, RAxML, and maximum parsimony were merged and collapsed (topology PP ≥ 0.8) in TreeGraph (Stöver & Müller, 2010). Final editing was completed in Adobe Illustrator 2021 v. 25.2 (Adobe Systems, Inc., San Jose, CA, USA).
Exploratory species delimitation analyses and uncorrected pairwise distance (p-distance) were performed to corroborate the identification of genetic species. The pairwise distance was estimated for the single gene COI using MEGAX v. 10.2.4 applying the p-distance model (Kumar et al., 2016). Assemble Species by Automatic Partitioning (ASAP) was applied to identify species hypothesis independently of their phylogenic reconstruction. This analysis was designed to apply the concept of “barcode gap” in single gene alignments. We ran ASAP using the COI and 16S alignments separately applying Kimura (k80) distance (ts/tv = 2.0), through the web interface (https://bioinfo.mnhn.fr/abi/public/asap/asapweb.html) (Kekkonen et al., 2015). In addition, the Bayesian Poisson tree process (bPTP) was performed to provide species hypothesis based on a phylogenetic approach. For that, we used the nexus files resulting from the concatenated ML analysis excluding the outgroups analysis (Zhang et al., 2013). The latter was run on the online bPTP web server (http://species.h-its.org/ptp/) (Heidelberg Institute for Theoretical Studies, Heidelberg, Germany) applying 500,000 generations with the remaining sets as defaults. Furthermore, a haplotype network analysis implemented in PopArt software was used to infer the genetic relationships of the different haplotypes applying TCS network. For this analysis, the COI alignment was used and trimmed to remove unknown nucleotides (N). The final alignment held 591pb; the sequence of H. lacer NM2014H1 from China was excluded for being too short (559), as well as H. lacer from GB as we were unable to trace the sample location.
Results
Phylogenetic and species delimitation analyses
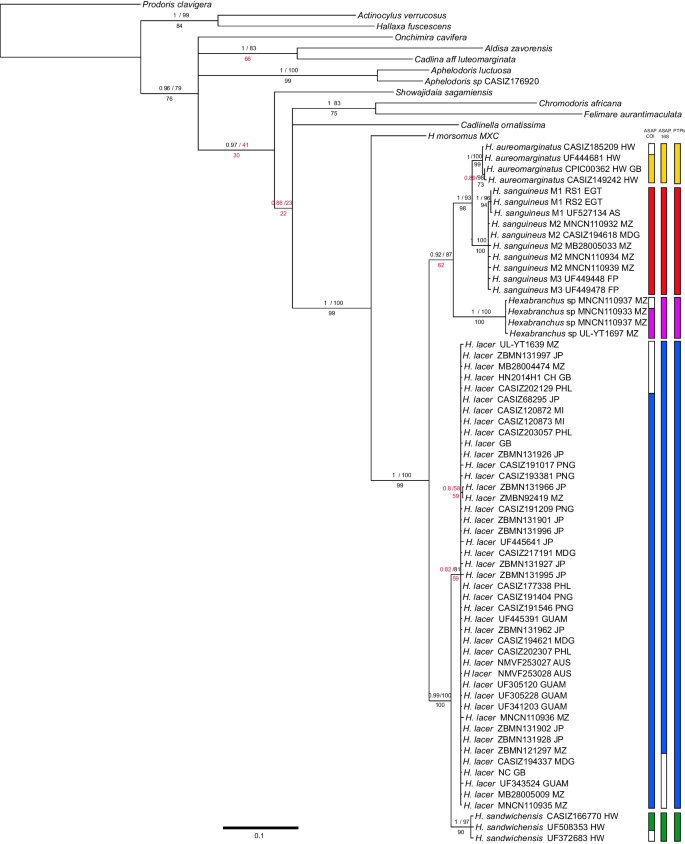
The three phylogenetic analyses yielded similar topologies; however, the relationship within the genus was better resolved by the RAxML analysis. The monophyly of the genus Hexabranchus was recovered by all phylogenetic analyses (PP = 1, BS = 100, MP = 99; Fig. 1), and in all of them, the Caribbean species Hexabranchus morsomus was recovered as a sister to the Indo-Pacific Hexabranchus species (PP = 1, BS = 100, MP = 99). The clade containing the Indo-Pacific species was divided into two main sub-clades. One is well supported (PP = 0.99, BS = 100, MP = 100), with a sub-clade containing the three specimens of H. sandwichensis (PP = 1, BS = 97, MP = 90) and a second sub-clade with all H. lacer specimens. The latter division was only supported by the RAxML analysis (PP = 0.82, BS = 81, MP = 52). The second major clade was strongly supported by the RAxML and maximum likelihood analysis, but moderately supported by maximum parsimony (PP = 0.92, ML = 87, MP = 62). This clade was divided into two sub-clades: a strongly supported clade with a sub-clade of H. aureomarginatus (PP = 1, BS = 100, MP = 99) and its sister sub-clade of H. sanguineus (PP = 1, BS = 100, MP = 100); and a second clade with maximum support containing all specimens of an undescribed species (PP = 1, BS = 100, MP = 100).
Bayesian phylogenic hypothesis represented by the collapsed phylogenetic tree (PP ≥ 0.5) of the genus Hexabranchus based on concatenated molecular data (COI + 16S + H3). Values on the top of the branches represent Bayesian posterior probabilities (PP, top left) and maximum likelihood bootstrap percentages (ML, top right) and on the bottom bootstrap values for maximum parsimony (MP). Colored bars indicate specimens grouped as species by the delimitation analyses, from left to right: ASAP based on COI gene, ASAP based on 16S gene and bPTP, based on the results of concatenated ML analysis excluding the outgroups. Empty bars on single-gene analysis (ASAP) show missing sequences
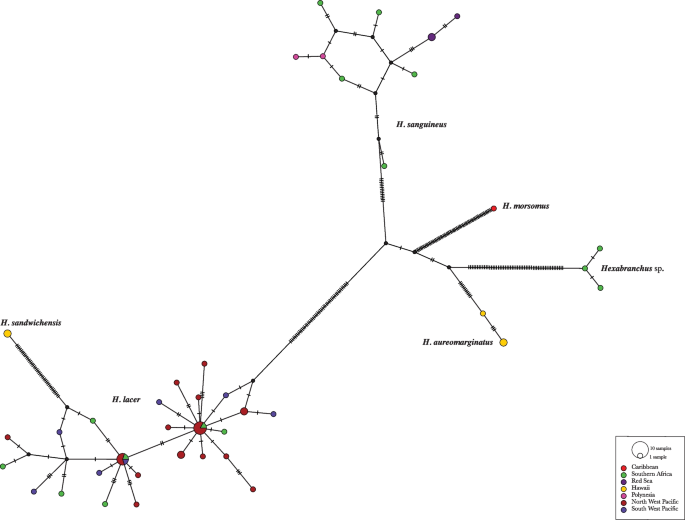
The three species delimitation analyses yielded the same results, splitting the specimens into six groups in accordance with the phylogenetic reconstruction (Fig. 1). This result is consistent with the COI haplotype network analysis, which shows several mutations between the six suggested species (Fig. 2). According to this analysis, H. sandwichensis shares a common ancestral with H. lacer, while the undescribed Hexabranchus species shares a common ancestral with H. aureomarginatus. Hexabranchus lacer is distributed into two major groups of haplotypes, but both contain specimens from the Pacific and Southern Africa. The minimum interspecific genetic distance was found between H. lacer and H. sandwichensis(6.99%) and the maximum between H. morsomus and H. giganteus sp. nov. (15.65%). Intraspecific variation ranged from null to approximately 2% (Table 3).
The haplotype network based on cytochrome c oxidase subunit I (COI) molecular data showing genetic mutations occurring within species of the genus Hexabranchus. Each hatch line represents one mutation and black dots represent hypothetical haplotypes. Each colored circle represents a unique haplotype and the size is relative to the number of specimens sharing the same haplotype. Different colors represent different regions
Systematics
Order Nudibranchia Cuvier (1817)
Superfamily Chromodoridoidea Bergh (1891)
Family Hexabranchidae Bergh (1891)
Genus Hexabranchus Ehrenberg (1831)
Hexabranchus Ehrenberg (1828−1831) [1831]: type species (by subsequent designation of Gray, 1847): Hexabranchus praetextus Ehrenberg (1828)
Synonym
Heptabranchus A. Adams (1848: 59)
Rhacodoris Mörch (1863). Mörch (1863: 54)
Aethedoris Abraham (1877: 237)
Albania Collingwood (1881: 133)
Caribranchus Ortea et al. (2003: 24)
Diagnosis
An amended diagnosis is here proposed: large dorid nudibranchs; soft in texture; devoid of spicules; extended mantle; lamellate rhinophores slightly bent back; gill branches multi-pinnate and contractile (not retractile); anal papilla elevated, central or sub-central and located within the gill circle; kidney pore near anus; two large, fleshy oral tentacles; large blood gland; radula with numerous hamate teeth; unarmed, elongated, coiled penis; capable of swimming with dorso-ventral undulating movement.
Hexabranchus lacer (Cuvier, 1804) (Figs. 3, 4, 5, 6 and 7)
Doris lacera Cuvier (1804) [August] (original combination): v.4, pgs. 453–465, 473, pl. 73, figs. 1–3b–c. Type locality: “la mer des Indes.” Declared “nomen oblitum” under ICZN Art. 23.9 versus Doris sanguínea Rüppell and Leuckart (1828) “nomen protectum” by Valdés (2002).
Hexabranchus lacer (Cuvier, 1804). Abraham (1876: 135) (new combination reference).
Doris marginata Quoy and Gaimard (1832: pgs. 255–256, pl. 17, figs, 1–5). Type locality: “Amboine” (now Ambon, Ambon Island, Indonesia). (new synonym)
Doris flammulata Quoy and Gaimard (1832: pgs. 257–258, pl.17, Fig. 6–8). Type locality: Friendly Islands, Tonga. (new synonym)
Heptabranchus burnettii Adams (1848, 1858). Plates. 63, Fig. 10, pg. 59]. Type locality: Borneo. (new synonym)
Hexabranchus adamsii: v. 3, pl. 219, Fig. 1. Type locality: Borneo. (new synonym)
Doris superba Gould (1852: 12: p. 301, pl. 23, figs. 396, 396a–c, 1856). Type locality: Fangasai Bay, Tutuilla, Samoa Island. (new synonym)
Doris sumptuosa Gould, 1852: Gould (1852): p. 303, pl. 24, figs. 398, 398a, 1856. Type locality: Friendly Islands, Tonga. (new synonym)
Doris gloriosa Kelaart (1858: v3 (1), pgs. 91–93). Type locality: Fort Frederick, Trincomalee, Sri Lanka. (new synonym)
Aethedoris indica Abraham (1877: p. 237). Type locality: Madras, India (new synonym)
Hexabranchus orbicularis Abraham (1877: pgs. 261–262, pl. 30, figs. 23–24). Type locality: Mauritius. (new synonym)
Hexabranchus anaiteus Bergh (1878: v4, p.73). Type locality: New Hebrides Islands, Vanuatu. (synonymized by Bergh, 1900: pg. 225)
Hexabranchus faustus Bergh (1878: v.2 (13): 550–555, pl. 41, Fig. 3: pls. 61, Figs. 14, 15: pls. 62, figs. 25–28; pl. 63, figs. 1–9; pl. 67, figs. 3–6). Type locality: Aibukit, Palau Islands. (new synonym)
Hexabranchus punctatus Bergh (1905: v.50, p. 92, pl. 12, Fig. 27). Type locality: Pulu-Kebala-dua, Borneo Bank (Sta. 79) W. of Celebes, Indonesia. (new synonym)
Distribution
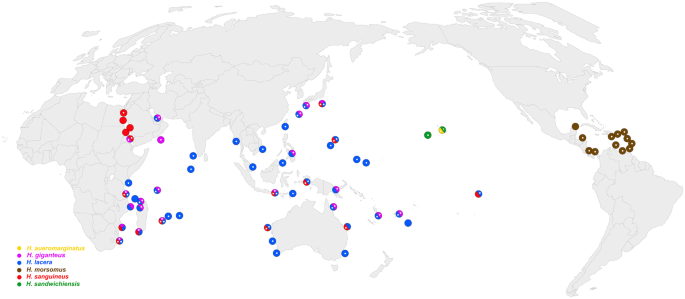
Broadly distributed across the Indo-Pacific from the western Indian Ocean to the central Pacific and French Polynesia: Oman (Debelius & Kuiter, ), Tanzania (Edmunds, ), Mozambique (Tibiriçá et al., 2017), Madagascar (present study), South Africa (Gosliner, 1987), India (Apte & Salahuddin, 2010), Thailand (Chavanich et al., 2010), Japan (Atsushi, 1999; Baba, 1936; Nakano, 2004), Philippines (Colin & Arneson, 1995), Indonesia (Debelius, 1996; Gosliner et al., 2008), Vietnam ( Debelius & Kuiter, 2007), Papua New Guinea (Coleman, 2008), Australia (Nimbs & Smith, 2016; Thompson, 1972) including Lord Howe Island (Coleman, 1989, 2001, 2008), New Caledonia (Hervé, 2010), Mariana Islands, Guam (present study), French Polynesia (Salvat & Bacchet, 2011), Tonga (present study). On-line sources add: Kenya, Comores, Mayotte, Emirate Arab, Maldives, Okinawa (Japan), Malaysia, Taiwan, East Timor, Marshall Islands, Vanuatu (iNaturalist), Reunion, Seychelles, Rodriguez, Mauritius (South-west Indian Ocean Seaslug site, 2011).
Material examined
Thirty-three specimens. CASIZ217191, length 35 mm (preserved), Philppines, Visayas, Siquijor Island, Paliton Wall (9° 10′ 12″ N, 123° 27′ 36″ E), 0–2 m depth, 4 Apr. 2016. CASIZ193381, length 11 mm (preserved), Papua New Guinea. CASIZ191209, length ≈20 mm, Papua New Guinea, Madang Province, 14 Nov. 2012. CASIZ191385, length ≈25 mm, Papua New Guinea, Madang Province, Sek Island, 22 Nov. 2012. CASIZ191017, length ≈30 mm, Papua New Guinea, Madang Province, Tab Island (5° 10′ 6″ S, 145° 50′ 31″ E), 7 Nov. 2012. ZMBN131962, length 15 mm, Japan, Hachijō-jima (33° 08′ 43″ N, 139° 44′ 18″ E), 5–19 m depth, 03 Oct. 2019. ZMBN131092, length 10 mm, Japan, Hachijō-jima (33°08′ 43″ N, 139° 44′ 18″ E), 5–19 m depth, 04 Oct. 2019. ZMBN131966, length 18 mm, Japan, Hachijō-jima (33° 08′ 43″ N, 139° 44′ 18″ E), 5–19 m depth, 04 Oct. 2019. ZMBN131997, length 105 mm, Japan, Hachijō-jima (33° 08′ 43.8″ N, 139° 44′ 18.6″ E), 7–22 m, 04 Oct. 2019. MB28-004474, length 67 mm, Mozambique, Zavora, rock pool (24° 31′ 09″ S, 35° 12′ 25″ E), 2 m depth, 7 Feb. 2012. UL-YT1639, length 80 mm, Mozambique, Ponta do Ouro, Atlantis (26° 50′ 58″ S, 32° 44′ 54″ E), 40 m depth, 16 April 2014. MHNM-0177 (MNCN:ADN 110935, tissue), length 220 mm, Mozambique, Nanatha Bay, Nuarro-Enupa (4° 12′ 03″ S, 40° 40′ 30″ E), 5 m depth, 31 Aug. 2017. NMVF253027, 24 mm length (preserved), Australia, Sunshine Coast, Caloundra, Raper Schoal (26° 23′ 26″ S, 153° 07′ 51″ E), 16 m, 16 Nov. 2018. NMVF253028 32 mm length (preserved), Australia, Sunshine Coast, Moloolaba Gneering Schoals (26° 23′ 10″ S, 155° 18′ 43″ E), 18 m depth, 16 Nov. 2018. CASIZ194337, length ≈30 mm, Madagascar, south Madagascar, ponte Evatra (24° 58.1′ S, 47° 6.1′ E), 3–8 m depth, 30 Apr. 2010. CASIZ68295, 38 mm (preserved), Ryukyu Island (24° 19′ N, 124° 10′ E), unknown depth, Mar. 1969. CASIZ191404, length ≈15 mm, Papua New Guinea, Madang Province (4° 35′ S, 145° 49′ E), 14 m depth, 23 Nov. 2012. CASIZ194621, length 60 mm (preserved), Madagascar, South Madagascar, Sud Ponte (24° 60′ S, 47° 6′ E), 18 m depth, 10 May 2010. CASIZ202307, length 31 mm (preserved), Philippines, Luzon, Batangas (13° 42′ 36″ N, 120° 52′ 12″ E), 0–2 m depth, 8 May 2014. MB28-005009, length 14 mm, Mozambique, Zavora, Area 51 (24° 26′ 28″ S, 35° 16′ 15″ E), 11 m depth, 12 June 2015. ZMBN131901, length 20 mm, Japan, Hachijō-jima (33° 08′ 43″ N, 139° 44′ 18″ E), 5–19 m depth, 03 Oct. 2019. ZMBN92419, length Mozambique, Nanatha Bay, Nuarro-Enupa (4° 12′ 03″ S, 40° 40′ 30″ E), 5 m depth, Mozambique. ZMBN131926, length 30 mm, Japan, Hachijō-jima (33° 08′ 43.8″ N, 139° 44′ 18.6″ E), 5–12 m, 03 Oct. 2019. ZMBN131927 length 75 mm, Japan, Hachijō-jima (33° 08′ 43.8″ N, 139° 44′ 18.6″ E), 5–12 m, 03 Oct. 2019. ZMBN131928, length 12 mm, Japan, Hachijō-jima (33° 08′ 43.8″ N, 139° 44′ 18.6″ E), 5–12 m, 03 Oct. 2019. ZMBN131967, length 13 mm, Japan, Hachijō-jima (33° 08′ 43.8″ N, 139° 44′ 18.6″ E), 5–19 m, 03 Oct. 2019. ZMBN131995, 4 spcs., length 6–17 mm, Japan, Hachijō-jima (33° 08′ 43.8″ N, 139° 44′ 18.6″ ″E), 7–22 m, 04 Oct. 2019. ZMBN131996, length 30 mm, Japan, Hachijō-jima (33° 08′ 43.8″ N, 139° 44′ 18.6″ E), 7–42 m, 04 Oct. 2019. ZMBN131997, 105 mm, Japan, Hachijō-jima (33° 08′ 43.8″ N, 139° 44′ 18.6″ E), 7–42 m, 04 Oct. 2019. CASIZ072156, 3 specimens., up to length 62 mm (preserved), Tonga, Nuku Island (18° 26′ 06″ S, 174° 01′ 12″ E), 1–5 m depth, 24 Jul. 1985. Other material: MNCN:ADN 110936 (tissue), length 400 mm, Mozambique, Ponta do Ouro (26° 50′ 6″ S, 32° 44′ 5″ E), 1 m depth, 16 April 2022.
External morphology (Figs. 3, 4 and 5)
Commonly up to 220 mm (with unconfirmed reports of 500 + mm in the Marshall Islands). The notum in resting, mature animals is broadly and irregularly pustulate. The body is pyriform, when the mantle is rolled, and oval when it is extended. Mantle extension becomes gradually wider toward the back but is short and differentiated on the head. The rhinophore sheath is short with a smooth edge. The peduncle is stocky, and the club is broader than in H. sanguineus. There are about 40–50 lamellae on the rhinophore clubs of large, mature animals. The gill branches are complex and multi-pinnate with a variable number of gill tufts (often more than seven) forming a circle around the anus. The anus is elevated on a tubular papilla. The kidney pore is on its right side. The oral tentacles are large, fleshy, oval, elongate, and crenate. The foot is narrower than the body.
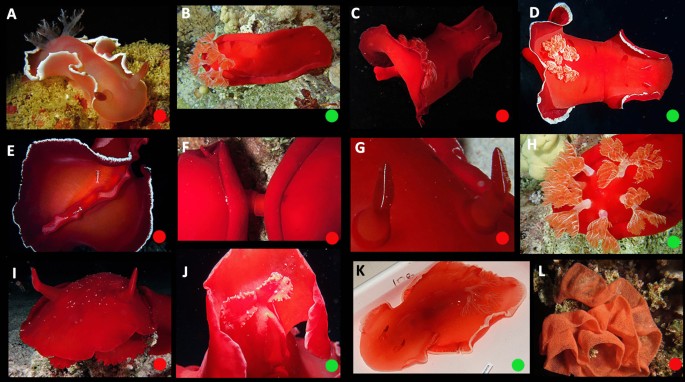
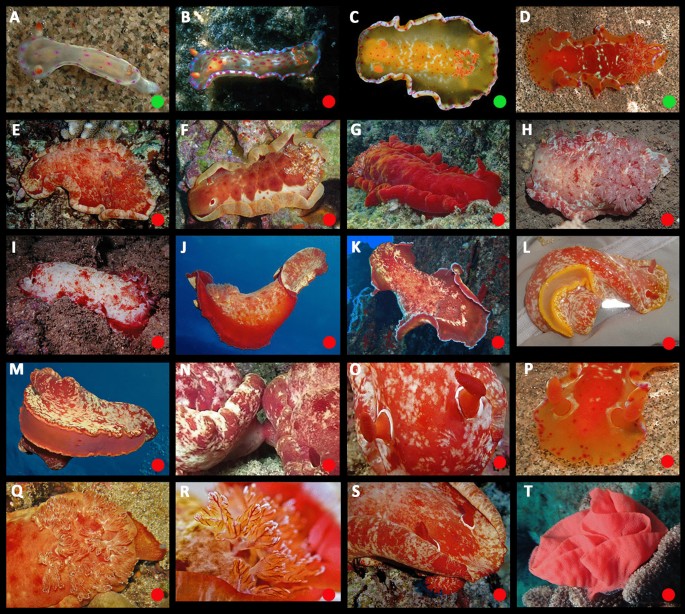
Ontogeny, color, and variation (Figs. 3, 4 and 5)
Hexabranchus lacer is abundant, widely distributed, and highly variable. The details of the dorsal banding vary with white marginal bands, red marginal bands, lateral striations, interruptions of the red band, and violet tinting “mixing and matching.” The innermost dorsal band of mature animals is sharply margined, medially, and strongly scalloped. Diffuse white pigment is often present on and around the rhinophore collars (most noticeable in larger animals) and the rhinophore lamellae are usually edged in white. There is a red line on the outer face of the rachis. The overall color pattern of mature animals is “mottled.”
There are two late-appearing traits that emerge after sexual maturity in some animals: “dark” and “cloudy.” In “dark,” the whole animal darkens, sometimes with complete replacement of the initial pattern. When present, the dark pigment is opaque (in contrast to translucent in dark-colored H. sanguineus). In “cloudy,” the dermis becomes partially opaque, obscuring the underlying pattern. This development is usually patchy allowing the underlying pigment to show through, irregularly, and sometimes creating the illusion of spots that are not inherent to the original pattern. The frequency of both morphotypes seems to vary between populations. Rarely, animals have extensive white pigment. The color of the foot sole is similar to the background color on the sides of the animal but with a pale margin. However, this pattern may be reversed or overridden in some very dark animals.
The maximum COI intra-specific genetic variation between all specimens was 1.98% (Table 3); no genetic structure or internal differences could be found between morphotypes. Despite that, extensive review of thousands of photographs suggests the presence of three distinct morphotypes that differ in their ontogeny.
Morphotype 1 (m1) (Fig. 3): (French Polynesia, central Pacific & western Pacific)
Juveniles lack purple spots in the center of the notum, purple marginal spots are present and typically larger than in morphotype 2 (m2), lack a white submarginal line, largely lack a translucent yellow marginal band on the front of the head, and appear to lose the white rhinophore bases earlier than in m2. Transitional animals develop a dense covering of white spots on all surfaces. In mature animals, the spots become larger and cluster to form closely spaced rosettes (either uniformly or patchily distributed). A diffuse white ring around the rhinophore collar is usually present, particularly in large animals, highlighting a narrow orange line on the margin of the collar. In “dark” animals, dark pigment fills in the space between the dorsal bands and the central notum but does not fully obscure the rosettes on the notum (or elsewhere). The “cloudy” trait is relatively rare. In mature animals, the mantle margin in all examined photos was white-banded and “tinted,” usually with lateral striations.
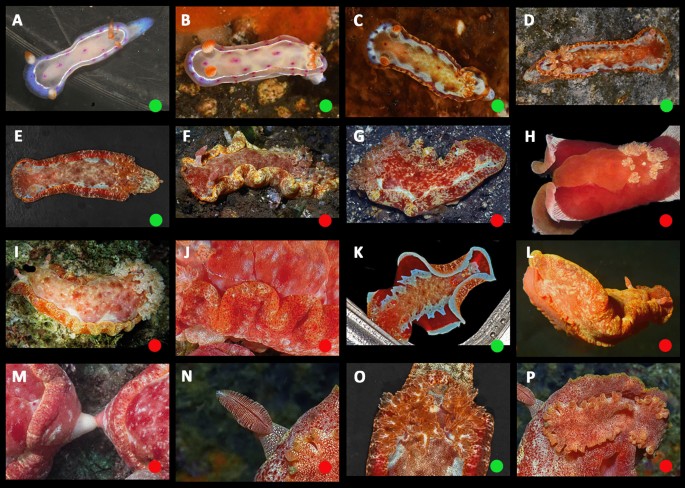
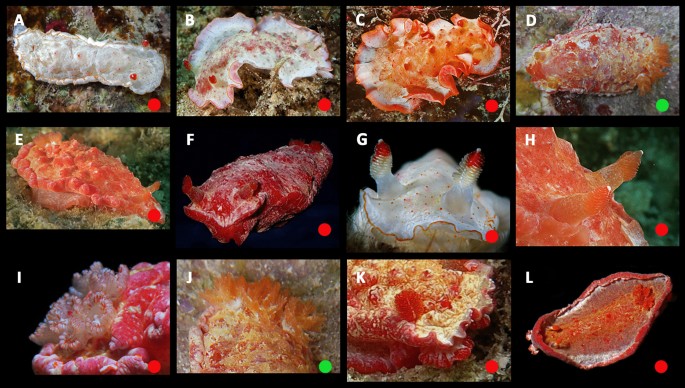
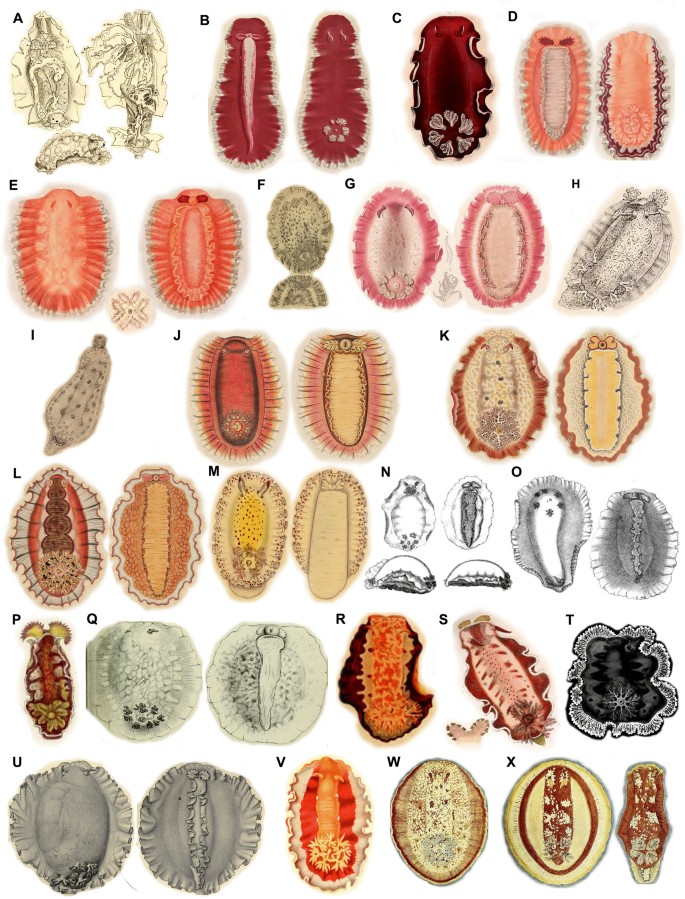
Ontogeny, color, and variation in Hexabranchus lacer (morphotype 1: Pacific). Green circles indicate studied specimens and red online sources. A early juvenile, B juvenile, C early transitional, D mild-transitional, E late transitional, F mature, even rosettes, G rosettes detail H mature, patchy rosettes. Imature dark, red J mature, dark, brown, K residual rosettes in large animal L mantle unrolled, white margin M rhinophores, N gills, O oral tentacles, P foot
Morphotype 2 (m2) (Fig. 4): (from western Pacific to Marshall Islands)
Juveniles have purple spots in the center of the notum, a submarginal white line, and white rhinophore base. Late juveniles and transitional animals have a translucent-gold band on the front of the head. In transitional animals, fine white flecks develop on the notum while the underside of the mantle becomes covered with small, diffuse red spots. Those two features appear to be strongly correlated but both may be either uniformly or patchily distributed. Some large animals develop the “dark” trait. First, the space between the lateral bands and the central notum apparently fills in with dark pigment. Then, the central notum fills in with moderately dark to dark pigment, obscuring the underlying pattern. Finally, the underside of the mantle fills in with dark pigment obscuring the red spots. The diffuse white rings around the rhinophore collars often remain visible in older animals but the dark pigment may completely replace other white features or reduce them to a few scattered, minute flecks. Moderate numbers of old animals develop the “cloudy” trait. White animals are rare. In mature animals, the margin may be either white-banded or red-banded, but “tinting” is probably somewhat less common than in the other morphotypes. The red band may be interrupted, and lateral striations may be present.
Ontogeny, color, and variation in Hexabranchus lacer (morphotype 2: Eastern and Western Pacific). Green circles indicate studied specimens and red online sources. A early juvenile, B juvenile, C late juvenile, D early transitional, E transitional, F mature, even flecks, G mature, patchy flecks, H mature, dark, I mature, cloudy J detail on spots on mantle underside K mature, unrolled, white margin Lunderside M genital papillae N rhinophores O gills P oral tentacles
Morphotype 3 (m3) (Fig. 5) (west Indian Ocean from Oman and Red Sea to the Maldives and south to Madagascar and South Africa)
Juveniles are nearly identical to juveniles of m2. They have purple spots in the center of the notum, a submarginal white line, and a white rhinophore base. Late juveniles and transitional animals have a translucent-gold band on the front of the head. In transitional animals, fine white flecks may develop on the notum while the underside of the mantle may become covered with small, diffuse red spots. Mature animals appear to develop more prominent yellow-white patches than in m2, both on the notum and on the underside of the mantle. The patches on the notum appear to form through “coalescence” of the white flecks while the patches on the mantle seem intrinsic to the underlying color. In “dark” animals, dark pigment replaces most of the patches although a few scattered remnants are sometimes retained, and a few even darker patches may develop on the notum. Many larger animals develop the “cloudy” trait. In some animals, red spots on the underside of the mantle may appear to be generated by the uneven distribution of cloudy pigment rather than being intrinsic to the underlying pattern. White animals are rare. In mature animals, the margin may be either red-banded or white-banded. “Tinting” lateral striations and interruptions of the red band are common.
As in other Hexabranchus spp., during ontogeny, the mantle expands laterally and becomes rolled, the number of rhinophore lamellae increases, the gills become more elaborate, and the notum of resting animals assumes the mature texture. The mantle margin in large animals may become somewhat frillier than in other species (highly dependent on posture).
Ontogeny, color, and variation in Hexabranchus lacer (morphotype 3: west Indian Ocean from Oman and Red Sea to the Maldives and south to Madagascar and South Africa). Green circles indicate studied specimens and red online sources. A Very early juvenile, B early juvenile, C late juvenile, D early transitional, E transitional, F transitional, unrolled mantle, G mature, H mature, dark I mature, cloudy pink J mature, cloudy dark K mature, cloudy red, L mature, cloudy white, unrolled mantle interrupted red band, M mature, light dorsum, unrolled mantle, red margin, N intermediary, unrolled mantle, yellowish margin, O underside dark, P underside light, Q rhinophores, R gills, S oral tentacles, T eggs
Internal morphology
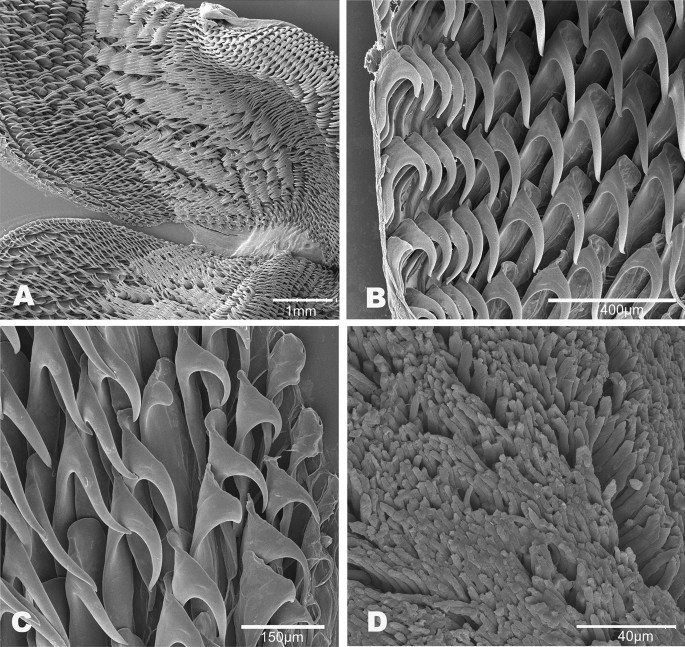
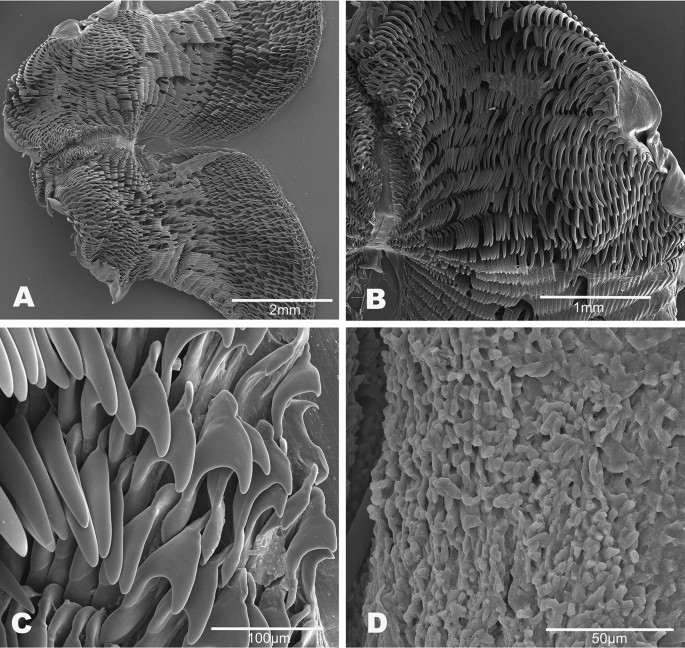
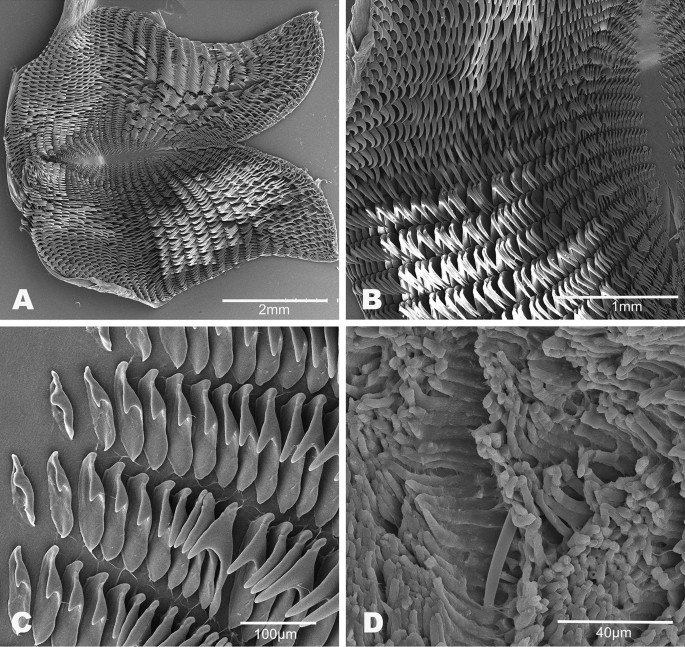
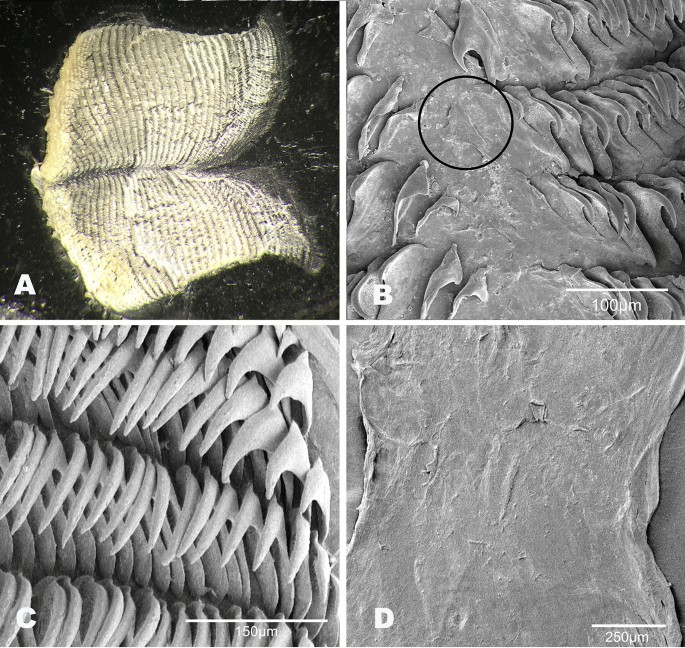
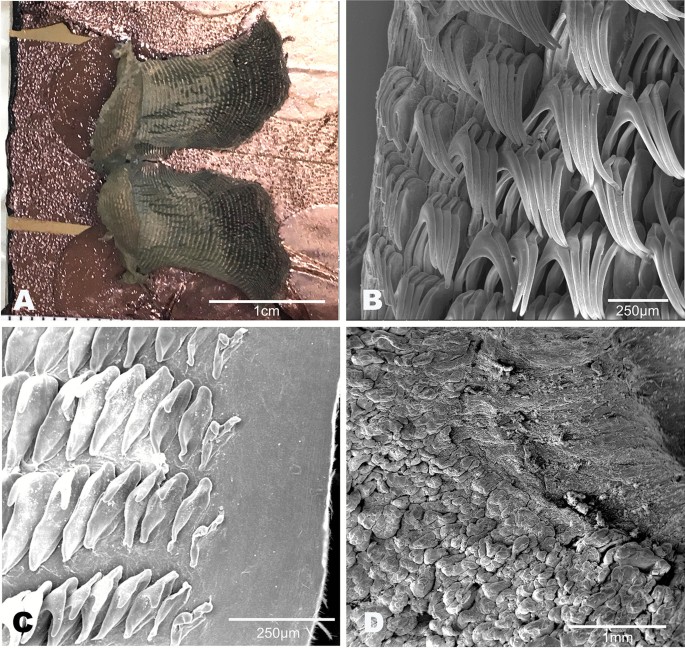
Buccal mass (Fig. 6). The buccal bulb is oval and slightly larger than the oral tube. The radula is broad and bi-lobed with the center of the ribbon devoid of teeth (Fig. 6A). The teeth are simple and hamate. The lateral teeth increase in length toward the center of the row. The outermost teeth are smaller or degenerate (Fig. 6B). The inner 4–9 teeth tend to lay laterally. The innermost teeth are smaller, degenerate, or vestigial (Fig. 6C). The radular formulae are 32 × 61.0.61 (CASIZ 193,381), 31 × 47.0.47(CASIZ 202,307), 35 × 55.0.55 (CASIZ 217,191), 33 × 47.0.47 (CASIZ 194,621), 30 × 41.0.41 (UF 253,028), 30 × 36.0.36 (UF 253,027), and 51 × 69.0.69 (MHNM-0177). The jaws are armed with numerous simple, finger-like rodlets (Fig. 6D).
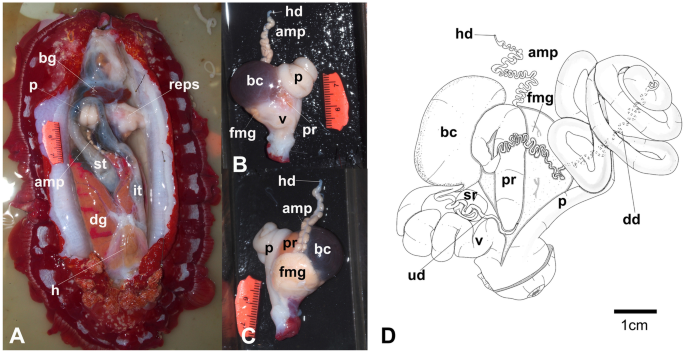
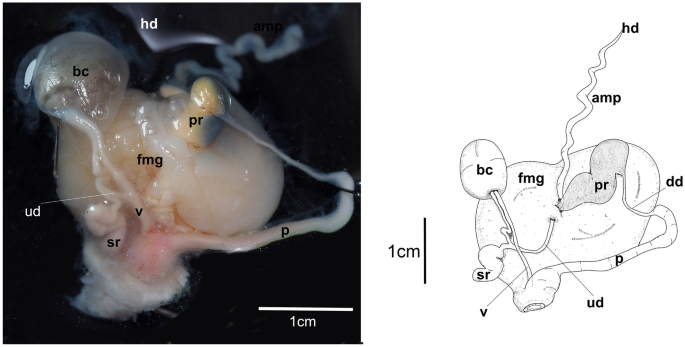
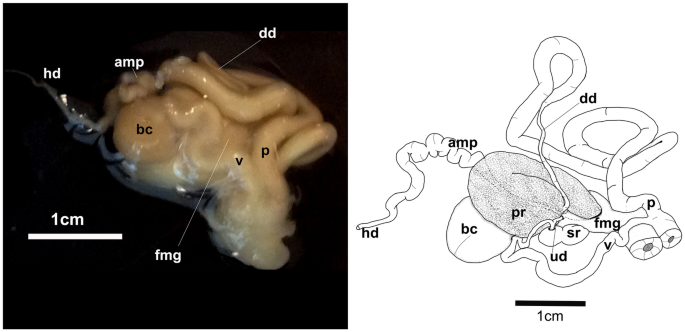
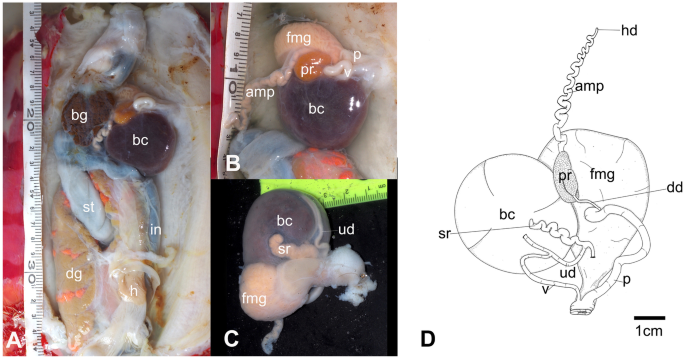
A fresh photograph of the internal morphology from a dissection of this species is illustrated here for the first time (Fig. 7A). The large blood gland is dark-brown covering part of the nerve ring. The intestine and stomach are bluish and of similar diameter. The digestive gland is cone-shaped and orange bearing a distinct pink duct.
Reproductive system (Fig. 7B–D)
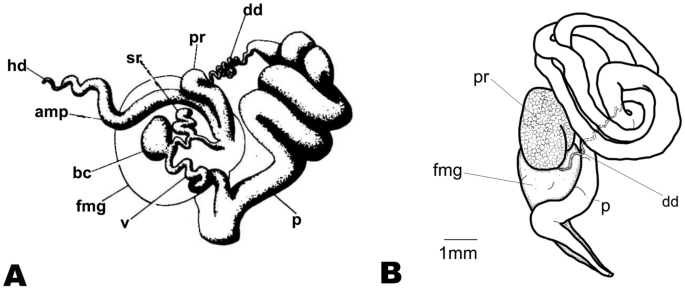
The reproductive system is triaulic with the hermaphroditic duct leading to a very long, convoluted, cream-colored ampulla. The ampulla divides into a short oviduct leading into the female gland mass and deferent duct through the prostate portion. The prostate gland is granular, kidney-shaped, orange in color, stocky at its base, and narrowing toward the distal deferent duct. The distal deferent duct is thin and long. The long, thick penis is coiled around the deferent duct. The deferent duct opens into a common atrium with the vagina. The vaginal duct is curved, thick, and enclosed by connective tissue. The vaginal duct bifurcates into ducts leading to the ventral side of the bursa copulatrix and the receptaculum seminis. The receptaculum seminis is short, convoluted, and creamy-orange. The bursa copulatrix is black, large, and oval. The short uterine duct emerges between the receptaculum seminis and the bursa copulatrix, entering the female gland. The female gland is oval, creamy in color, and of similar size to the bursa copulatrix.
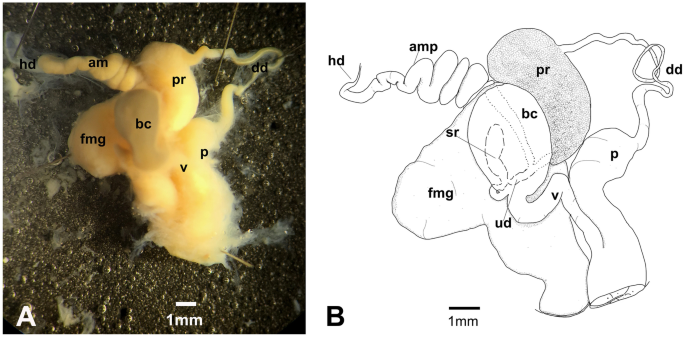
Hexabranchus lacer. A Photography of internal anatomy of exemplar MHNM-0177 with details on the reproductive system (ventral view above and dorsal view below). B Reconstructed drawing. Abbreviations: amp, ampulla; bc, bursa copulatrix; bg, blood gland; dd, deferent duct; dg, digestive gland; fmg, female gland; h, heart; hd, hermaphroditic duct; it, intestine; p, penial bulb; pr, prostate; reps, reproductive system; rs, receptaculum seminis; st, stomach, ud, uterine duct; v, vagina
Natural history and behavior
Francis (1980) found that, in Tonga, sightings of this species were affected by tidal period and their habitat preference depended on their stage of development. He also pointed out that “the animals actively avoided live coral” (page 254). Apte and Salahuddin (2010) found that 200 mm specimens were active at night at low tide. In Mozambique, juveniles and intermediary specimens are commonly seen in tidal pools and shallow water. Larger specimens are usually found below 5 m. Particularly in the north of Mozambique where the predominant habitat is coral reefs, which offers more hidden spaces than in the southern rocky reefs, this species is only found at night.
Edmunds (1968) suggested a difference in swimming behavior between H. lacer (as H. marginatus) and H. sanguineus but, as pointed out by Thompson (1972), this difference is barely discernable. Additional information on the reproduction and feeding behavior of this species is provided by Francis (1980).
Remarks
as predicted by Edmunds (1968), our results show that H. lacer and H. marginatus are the same species, which is distinct from H. sanguineus.
No genetic, behavioral, or internal morphological differences could be found to differentiate the three studied morphotypes of H. lacer, suggesting that they are likely variations of the same highly polychromatic species. Nevertheless, the existence of distinct morphotypes may provide a starting point for further investigation. We have found photos of 27 sets of copulating or closely paired animals (two of m1, 23 of m2, and two of m3). Several of these pairs were light and dark animals but none of them cross the boundaries between the morphotypes. That might suggest that m1 and m2 are reproductively isolated even though they are largely sympatric. In addition, Morton (1964) described the swimming behavior of a specimen of m1 which differed from the swimming behavior described by Edmunds (1968) for a specimen of m3. Edmunds (1968) suspected that such a difference might indicate different species, but this could not be confirmed.
Relative to the sympatric H. sanguineus, the egg mass is usually higher, more tightly coiled, and darker in color. The gills of H. lacer are typically held in a more recumbent posture than in H. sanguineus. In H. lacer, the notum appears broadly and irregularly lightly to strongly pustulate in large, resting animals. Nevertheless, the pustules largely “disappear” during swimming. The genital papillae appear to have tapered margins in copulating pairs. Juveniles and transitional animals are commonly seen in tide pools and shallow water, while mature animals are found in moderately protected submerged habitats and on deeper reefs.
Hexabranchus sanguineus (Rüppell & Leuckart, 1830) (Figs. 8, 9, 10, 11, 12, and 13)
Doris sanguinea Rüppell and Leuckart (1828–1830) (original combination): pgs. 28–29, pl. 8, Fig. 1. Type locality: Tor, Egypt, Gulf of Suez. Declared “nomen protectum” by Valdés (2002)
Hexabranchus sanguineus (Rüppell & Leuckart, 1830). Abraham (1876: pgs. 103–108) (new combination reference).
Hexabranchus praetextus Ehrenberg (1828: pt1-2, pl. 1a-c). Type locality: El Tur, Egypt (synonymized by Thompson, 1972).
Hexabranchus suezensis Abraham (1876: v. (4) 18, pgs. 137–138, pl. 6, figs. 3, 3a). Type locality: Red Sea (synonymized by Thompson, 1972).
Hexabranchus petersi Bergh (1878: 2 (13), pgs. 60–564, pl. 64, Fig. 1; pl. 67, figs. 7–9). Type locality: Quirimba Islands, Northern Mozambique, East Africa (synonymized by Valdés, 2002).
Albania formosa Collingwood (1881: v.2 (2), p.133, pl. 10, Figs. 1–5). Type location: Ke-lung Harbour, Formosa, Taiwan (synonymized by Thompson, 1972).
Hexabranchus plicatus Hägg (1901: v.6, pgs. 5–7, pl. 1, figs. 4–5). Type locality: Tor, Egypt. (synonymized by Thompson, 1972).
Material examined
CASIZ194618, length 75 mm (preserved), Madagascar, Sud Baie de Lokaro (24° 57′ S, 47° 6.5′ E), 10 m depth, 12 May 2010. MB28-005033, length 250 mm, Mozambique, Ponta do Ouro (26° 50′ 58″ S, 32° 44′ 54″ E), 39 m depth, 18 June 2016. MNCN:ADN 110932 (tissue), length ≈250 mm, Mozambique, Ponta do Ouro (26° 50′ 58″ S, 32° 44′ 54″ E), 40 m depth, 10 May 2014. UF455939 Jeddah, Saudi Arabia (21° 45′ 24.1″ N, 39° 03′ 06.5″ E), 15 m depth, 9 October 2012, collected by Gustav Paulay. MNCN:ADN 110934, length 240 mm, Mozambique, Ponta do Ouro, “The Cake” (26° 50′ 22″ S, 32° 54′ 39″ E), 38 m depth, 12 April 2022. Other material: MNCN:ADN 110939 (tissue), length ≈250 mm, Mozambique, Ponta do Ouro (26° 50′ 58″ S, 32° 44′ 54″ E), 40 m depth, 17 Nov. 2018. UF449478 (tissue), French Polynesia, Marquesas Islands, Fatu Hiva Island (10° 31′ 58.4″ S, 138° 41′ 05.6″ W), 3 Dec. 2011 (FLMNH Invertebrate Zoology). UF449478 (tissue), French Polynesia, Marquesas Islands, Fatu Hiva Island (10° 31′ 58.4″ S, 138° 41′ 05.6″ W), 3 Dec. 2011 (FLMNH Invertebrate Zoology). Sequenced but not deposited (tissue), length ≈250, Red Sea, Global Range (27° 40′ 07″ N, 33° 48′ 32″ E), 10 m depth, 29 Oct. 2018. Sequenced but not deposited (tissue) length ≈230 mm, Red Sea, Egypt, Shaab Samadai East (24° 59.144′ N, 34° 59.798′ E), 10 m depth, 1 Nov. 2018.
Distribution
Broadly distributed across the Indo-Pacific from the Red Sea to French Polynesia: Egypt (Debelius, 1996; Rüppel & Leuckart, 1828; Yonow, 2008), Sudan (Debelius, 1996), Sri Lanka (Debelius, 1996), southern Mozambique (Stromvoll & Jones, 2019; Tibiriçá et al., 2017), South Africa (Gosliner et al., 2008; King & Fraser, 2014), Seychelles (Debelius, 1996), Japan (Atsushi, 2004; Nakano, 2004), and Australia (Marshall & Willan, 1999) including Lord Howe Island (Coleman, 2001, 2008), New Caledonia (Hervé, 2010), and French Polynesia (Salvat & Bacchet, 2011). On-line sources: Israel, Tanzania (iNaturalist), Indonesia (Sea Slug Forum) including West Papua (iNaturalist), Madagascar, Reunion (South-west Indian Ocean Seaslug site), north-western Australia (Sea Slug Forum, iNaturalist), east Australia (Nudibranchs Sunshine Coast Queensland, Australia), Saipan (Nudipixel archive).
External morphology (Figs. 8, 9, 10, and 11)
Commonly up to 250 mm (with some reports to 400 mm in the Red Sea). The body of resting, mature animals is smooth and more dorsal-ventrally compressed than in other Hexabranchusspp. The extended mantle has an undulating edge that is very thin and delicate on the sides, posteriorly, but shorter and thicker with a smooth edge, anteriorly. The rhinophore sheath is very short with smooth edges. The rhinophores are slightly bent to the back with approximately 40 lamellae in large, mature animals. There are usually six tufts of multi-pinnate gill branches set widely apart and forming a circle around the anus. The anus is elevated on a tubular papilla. The kidney pore is anterior to the anus on its right side. The oral tentacles are large, fleshy, oval, and crenate. The foot is narrower than the body.
Ontogeny, color, and variation (Figs. 8, 9, 10, and 11)
There are four, apparently disjunct, lineages that differ in color (which changes with ontogeny).
Lineage 1 (Fig. 8)
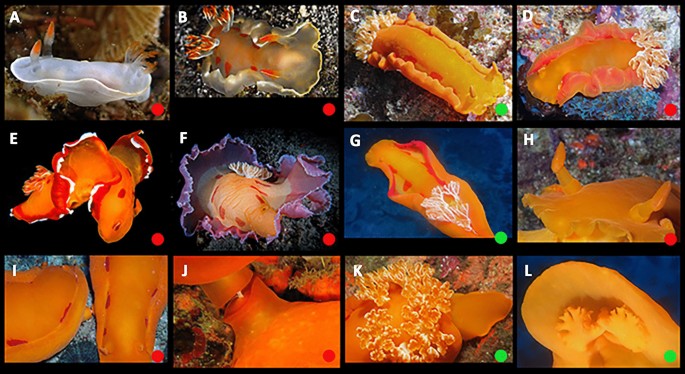
Predominantly in the Red Sea with very few records in the western Indian Ocean. No specimens or photos of very young juveniles were available. Gohar and Soliman (1963) provided information on intra-specific and ontogenetic variation in this lineage for specimens above approximately 75 mm, and it is in agreement with the photos reviewed in this study. Transitional animals vary from translucent pink to pink-reddish with variable white marginal bands. As the transition proceeds, lateral red patches (truncated medially) begin to develop, white pigment develops on the outer face of the rachis and often a white line appears on the anterior face of the rhinophore club. With growth, a submarginal red band develops. In large animals, the background usually darkens to blood red, which may obscure the lateral red markings. Additionally, a white marginal band is often present. A small amount of white pigment may appear on the posterior edge of the rhinophore stalk. There are no white markings on the notum or white flecks on the rhinophore lamellae. Lateral striations are uncommon.
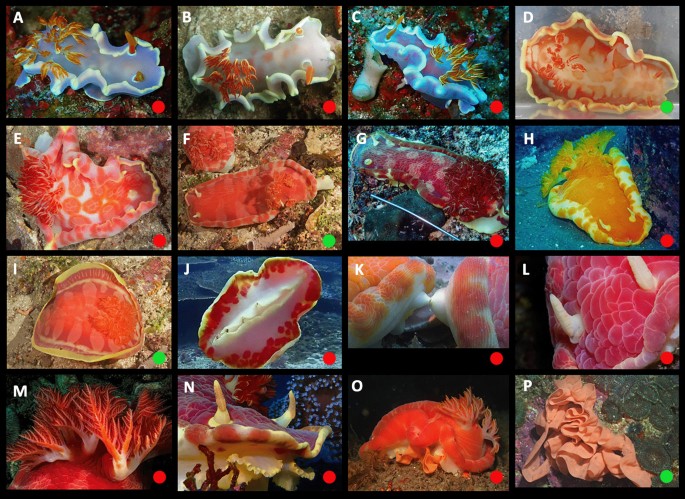
Ontogeny, color, and variation in Hexabranchus sanguineus (lineage 1). Green circles indicate studied specimens and red online sources. A early transitional, pink, B mature, C unrolled mantle, red margin, D unrolled mantle, white margin, E underside, F genital papillae, G rhinophores, H gills, I oral tentacles, J oral tentacle, pink margin, K transitional in laboratory, L egg mass
Lineage 2 (Fig. 9)
Western Indian Ocean (from Tanzania to South Africa). It is similar to lineage 1 in pattern and ontogeny, but the mantle color is predominantly orange. Young juveniles are gray with a marginal white line. The branchia and rhinophores are translucent-gray, tipped with orange-red. Transitional animals are grayish-yellow in color with a yellow-white edge, and a few orange-red lateral patches. The rhinophores are orange. Transitional animals are light orange with a marginal red band and, in some specimens, a white edge. As it grows, the mantle gets darker and the marginal band wider, while the lateral red patches (truncated medially) increase in number and size. The rhinophores remain orange and a white line often develops on the anterior face of the club. As in lineage 1, lateral striations are uncommon, and no white flecks are present on the notum or rhinophore lamellae.
Ontogeny, color, and variation in Hexabranchus sanguineus (lineage 2). Green circles indicate studied specimens and red online sources. A early juvenile B transitional C mature, D unrolled mantle, red margin, E unrolled mantle, white margin, F unrolled mantle, violet margin G unrolled mantle, red margin H rhinophores, I genital papillae, J genital papillae, close up, K gills, L oral tentacles
Lineage 3 (Fig. 10)
French Polynesia. No specimens or photos of juveniles were available but transitional animals appear to vary from translucent pink to yellow with extensive white pigment clustered in large rosettes. The rachis has dense white pigment. Dark lateral patches and red submarginal or marginal bands develop fairly late. Larger animals may become dark-red with some reduction in the white pigment and a white line may appear on the anterior face of the rhinophore club. The rhinophore lamellae are often flecked with white. The margin in mature animals may be either red-banded or white-banded with the former being most common.
Ontogeny, color, and variation in Hexabranchus sanguineus (lineage 3). Green circles indicate studied specimens and red online sources. A pink, transitional, B orange, transitional, C light, mature D dark, mature E unrolled mante, mature F underside, G gills, H rhinophores, I mature specimen in laboratory
Lineage 4 (Fig. 11)
Western Pacific. Regrettably, we could not obtain specimens of this morphotype for DNA analysis but based on extensive photograph review it is likely that a fourth distinct lineage of H. sanguineus exists in this region. This lineage shows an intermediate pattern between the ones from the Indian Ocean and the one from French Polynesia. Juveniles vary from translucent gray to yellow with a white marginal band and scattered white flecks on the notum. With growth, red lateral patches and a red submarginal band develop, white pigment appears on the rachis and the white flecks form clusters on the notum. The clusters of white flecks are present in almost all mature animals while the lateral patches are more variable than in specimens from the Indian Ocean. Dark-red animals occur but they appear to be limited to the northern and southern extremes of its distribution (southern Japan and Lord Howe Island, respectively). A white line usually appears on the anterior face of the rhinophore club. The rhinophore lamellae are often flecked with white. Pale animals seem rare. The margin in mature animals may be either red-banded or white-banded. Rarely, the red band may be interrupted (with associated lateral striations).
Ontogeny, color, and variation in Hexabranchus sanguineus (putative lineage 4). Red circles indicate online sources. A juvenile, B late transitional, C mature, light orange background, lightly flecked, Dmature, red background, moderately flecked, E swimming, white marginal band, F stranded, red marginal band, G mature pair, heavily flecked, H lighly flecked, detail, I heavily flecked, detail, J gills, juvenile, K gills, mature, L rinophores and oral tentacles
In all four lineages, during ontogeny, the mantle expands laterally and becomes rolled, the number of rhinophore lamellae increases and the gills become more elaborate. The notum of resting animals remains smooth.
Internal morphology
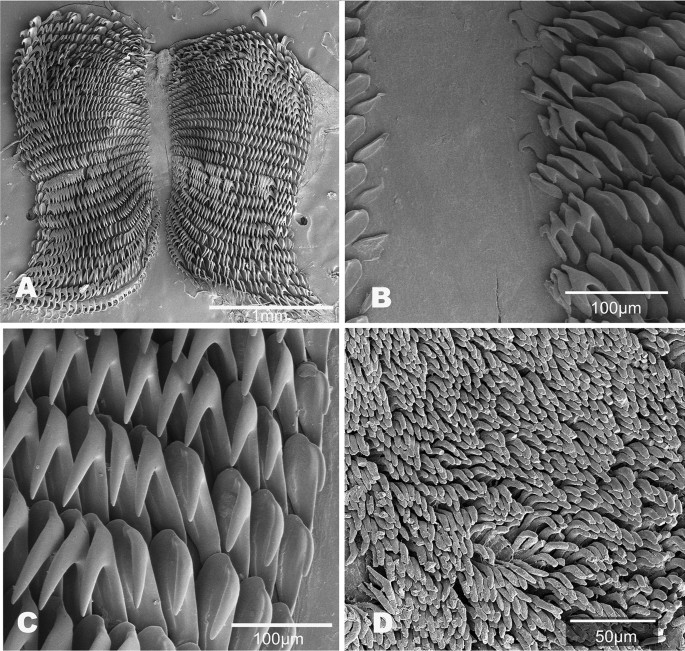
Buccal mass (Fig. 12). The buccal bulb is oval and slightly larger than the oral tube. The radula is broad and bilobed with 30 anterior raised rows of teeth (Fig. 12A). The center of the ribbon is devoid of teeth. The teeth are simple and hamate. The innermost teeth are smaller and degenerate. The lateral teeth increase in length centrally (Fig. 12B). The outer teeth are smaller (Fig. 12C). The radular formula is 49 × 89.0.89 (MB28-005033) and 32 × 50.0.50 (CASIZ194618). The armed jaws have numerous simple, finger-like rodlets (Fig. 12D).
Reproductive system (Fig. 13)
As described by Eales (1953) and Gohar and Soliman (1963).
Natural history and behavior
H. sanguineus can be found in shallow water and tide pools. Specimens from the Red Sea are night feeders, while specimens from the western Indian Ocean are more often seen feeding and mating during the day. In other parts of the Indo-Pacific, this species appears to be much rarer and information on its behavior is lacking. Nevertheless, it is unclear if these differences in abundance and behavior are an artifact of sampling (diving frequency/access). Titan triggerfish (Balistoides viridescens (Bloch & Schneider, 1801) has been seen feeding on this species (Ribes-Beaudemoulin et al., 2019).
Gohar and Soliman (1963) and Mahmoud and Raafat (2016) provide further insights into the behavior, larval development, and ecology of this species. The lack of photographs of small juveniles (for most populations) suggests that their larvae settle in locations not easily accessible to divers.
Remarks
H. sanguineus is the sister species of H. aureomarginatus, endemic to Hawaii, with a genetic COI divergence of 5.62% (p-distance) (Table 3). They share a similar number of gills, a smooth notum, and some color traits. Nevertheless, they differ from each other in some color details, distribution, and reproductive system. In H. sanguineus, the deferent duct and vagina are much shorter than in H. aureomarginatus. Relative to the sympatric H. lacer, the gills of H. sanguineus are typically held in a more erect position, the notum appears smooth in both resting and swimming mature animals and the genital papillae appear to have parallel margins in copulating pairs. The COI genetic divergence between them is very high (12.16%) and the reproductive system is clearly distinct, especially the short deferent duct in H. sanguineusversus the long and coiled deferent duct in H. lacer.
In agreement with the haplotype network, all specimens from the Red Sea nested together (PP = 1, BS = 96, MP = 94) (Fig. 1), but no clear distinction was found between the morphotypes from French Polynesia and southern Africa (Fig. 2). The maximum COI intra-specific genetic divergence was less than 2% (Table 3) and none of the species delimitation analyses recovered these morphotypes as distinct species (Fig. 1). This indicates that their differences likely reflect different populations. Contradictorily, the four lineages described above can be separated by color, geography, and some aspects of behavior. Regrettably, not all morphotypes were available for the molecular study and, in particular, we did not have access to any specimens of the fourth linage from the western Pacific. Because incomplete lineage sorting can hamper species delimitation analyses in recently diverged groups and the haplotype network reveals that our dataset is missing several haplotypes, we cannot fully reject the possibility that members of this clade represent recently diverged species instead of distinct populations. Additional material and more molecular markers would be necessary to clarify this hypothesis.
Hexabranchus sandwichensis (Gray, 1850) (Figs. 14, 15 and 16)
Hexabranchus sandwichensis (Gray, 1850. 3: 104, pl. 235 (original combination)). Type locality: Hawaiian Islands.
Doris sandwichiensis (Eydoux & Souleyet, 1852: v.2, pgs. 451–452. Pl. 25, figs. 1–4). Type locality: Hawaiian Islands.
Doris cardinalis (Gould, 1852: v.12, p. 302, figs. 397, 397a, b). Type locality: Honolulu, Hawaiian Islands. (new synonym)
Hexabranchus cardinalis (Gould, 1852) (new combination by Abraham, 1876: 135).
Hexabranchus pulchellus (Pease, 1860: v.33, pl. 28). Type locality: Sandwich Islands, Hawaii. (new synonym)
Hexabranchus tinkeri (Ostergaard, 1955: v9 (2), pgs. 128–130, pl. 2, text figs. 14a–e). Type locality: Waikki, Oahu, Hawaiian Islands. (new synonym)
Distribution
Restricted to the Hawaiian Islands and Johnston Atoll (Bertsch & Johnson, 1982; Debelius & Kuiter, 2007; Kay & Young, 1969).
Material examined
Five specimens. CASIZ167983, length 28 mm (preserved), Maui, HI, USA (20° 59′ N, 156° 40′ W), 9 m depth, 12 Sep. 2003. CASIZ116917, Oahu Island, HI, USA (21° 17′ N, 157° 57′ W), 10 m, at night, 10 June 1985. CASIZ166770, length 3 mm (preserved), Maui, HI, USA (20° 59′ N, 156° 40′ W), 1–4 m depth, 26 Apr. 2003. UF372683 (dissected and sequenced), length 15 mm (preserved), Napoli Bay, Maui, HI, USA (20° 59′ 39.8″ N, 156° 40′ 05.1″ W), intertidal pool, 12 December 2004. UF508353 (dissected and sequenced), length 14 mm (preserved), Honolulu, HI, USA (21° 28′ 48.0″ N, 157° 47′ 09.6″ W), 11–16 m depth, 26 May 2017.
External morphology (Fig. 14)
Commonly up to 300 mm. The notum in resting, mature animals is broadly and irregularly pustulate. The body is pyriform, when the mantle is rolled, and oval when it is extended. The mantle extension becomes gradually wider toward the back but is short and differentiated on the head. The rhinophore sheath is short with a smooth edge. The peduncle is stocky, and the club is broader than in H. aureomarginatus. There are about 40–50 lamellae on the rhinophore clubs of large, mature animals. The gill branches are complex and multi-pinnate with a variable number of gill tufts forming a circle around the anus. The anus is elevated on a tubular papilla. The kidney pore is on the right side of the anus. The oral tentacles are large, fleshy, oval, elongate, and crenate. The foot is narrower than the body.
Ontogeny, color, and variation (Fig. 14)
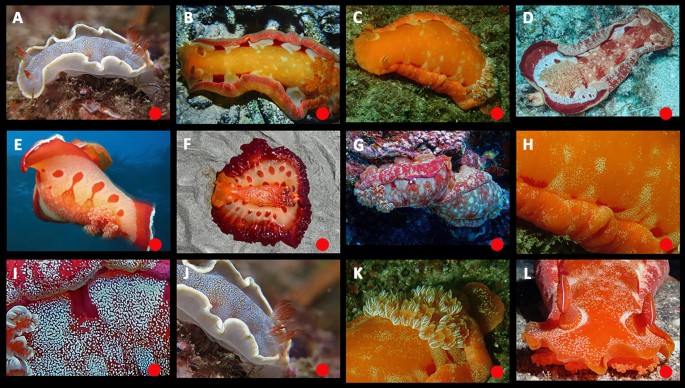
Juveniles have purple spots in the center of the notum and white rhinophore bases but lack a submarginal white line. Transitional animals lack a yellow band on the front of the head. The purple spots become reddish-purple and increase in number, with growth, while the complete background turns bright yellow. The notum then darkens to red with cream patches. A sharply defined white band develops on the rhinophore collar (in contrast to the diffuse band in H. lacer). As in H. lacer, “dark” and “cloudy” traits may develop in some animals after sexual maturity. The “dark” trait is fairly common and sometimes even obscures the white band on the rhinophore collar. The “cloudy” trait is rare in large animals. White animals are rare. Mature animals consistently have a red marginal band that is broad dorsally and narrow ventrally. Its border is diffuse on the dorsal side and well-defined on the ventral side. The mantle may have a translucent edge (without white pigment), when spread, and there may be weak lateral striations. The rhinophore lamellae are not edged in white. There is a red line on the outer face of the rachis.
Ontogeny, color, and variation in Hexabranchus sandwichensis (Hawaii). Green circles indicate studied specimens and red online sources. A juvenile, B early transitional, C mid-transitional, D late transitional, E mature, F mature, dark, G mature, very dark, H mature, cloudy, I mature, white, Jmature, unrolled mantle, K unrolled mantle, thinned margin, L underside, light, M underside, dark, Ngenital papillae, O rhinophores, mature, P rhinophores, young, Q gills, top, R gills, side, S oral tentacles, T egg mass
As in H. lacer, during ontogeny, the mantle expands laterally and becomes rolled, the number of rhinophore lamellae increases, the gills become more elaborate, and the notum of resting animals assumes the mature texture.
Internal morphology
Buccal mass (Fig. 15). The buccal bulb is of similar size to the oral tube. The radula is broad and bi-lobed (Fig. 15A) with the center of the ribbon devoid of teeth (Fig. 15B). The teeth are simple and hamate. The lateral teeth increase in length toward the center of the row. The outermost teeth are distinguishably smaller (Fig. 15C). The inner 6–12 teeth tend to lie laterally. The 1–3 innermost teeth are smaller, degenerate, or vestigial (Fig. 15C). The radular formula is 28 × 38.0.38 (UF508353 and UF372683). The jaws are armed with numerous simple, finger-like rodlets (Fig. 15D).
Reproductive system (Fig. 16)
Unfortunately, all examined specimens were immature. Nevertheless, the larger specimen (28 mm) showed signs of development with the prostate connected to a wide, long, coiled penis surrounding a thin, long, coiled deferent duct (Fig. 16A). Kay and Young (1969) provided a description of a mature reproductive system for this species (as H. marginatus) (Fig. 16B).
Hexabranchus sandwichensis reproductive system. A Reproductive system after Kay and Young (1969), B reconstructed drawing of immature specimen (CASIZ167983). Abbreviations: amp, ampulla; bc, bursa copulatrix; dd, deferent duct; fmg, female gland; hd, hermaphroditic duct; p, penial bulb; pr, prostate; rs, receptaculum seminis; ud, uterine duct; v, vagina
Natural history and behavior
It can be found in shallow water and tidal pools and is most common at moderately protected sites. It is primarily active at night.
Remarks
Up to now, H. sandwichensis has been considered a synonym of H. sanguineus, but nudibranch enthusiasts who frequently dive in Hawaii often disagreed with this and tentatively applied the name H. pulchellus to represent the species (e.g., Sea Slug of Hawai’i and MarinelifePhotography.com). The description of H. pulchellus is unmistakably of a juvenile H. sandwichensis. However, H. sandwichensis was described before H. pulchellusand therefore has priority over it. This species is closely related to the widely distributed Indo-Pacific species H. lacer, a fact noted by Eydoux and Souleyet in (1852) (as H. marginatus) and confirmed by our phylogenetic analysis. Their COI genetic divergence is about 7% (Table 3). They share similarly pustulate notum, a predominantly mottled appearance and similar reproductive system. Nevertheless, in comparing this species to H. lacer, H. sandwichensishas a red mantle border without striations and the vagina is considerably thinner. Relative to the sympatric H. aureomarginatus, the egg mass is usually higher, more tightly coiled, and darker in color. The gills are typically held in a more recumbent posture than in that species. The notum appears broadly and irregularly pustulate in large, resting animals but the pustules largely “disappear” when swimming. The genital papillae appear to have tapered margins in copulating pairs.
Hexabranchus aureomarginatus (Ostergaard, 1955) (Figs. 17, 18 and 19)
Hexabranchus aureomarginatus (Ostergaard, 1955) (original combination): v.9 (2): 132–133, pl. 2, text figs. 15a-f. Type locality: Waikiki, Oahu, Hawaiian Islands.
Material examined
CASIZ182737, approx. 65 mm, Kauai Island, Kiohuna Beach, HI, USA (22° 04′ 11.6″ N, 159° 18′ 50.5″ W), depth 1–3 m, 05 Oct. 2000. UF444681, (tissue), Kauai Island, Kiohuna Beach, HI, USA (22° 04′ 11.6″ N, 159° 18′ 50.5″ W), 13 May 2010. CASIZ142942, Maui, Kapalu Bay, HI, USA, 1–5 m, 05 Oct. 2000. CASIZ74634 (dissected), 70 mm (preserved), Kauai, Hawaii Islands, USA (22° 04′ 11.6″ N, 159° 18′ 50.5″ W), intertidal, 24 Feb. 1986. CASIZ 074271 (dissected), length 45 mm (preserved), Kauai Island, Kiohuna Beach, HI, USA (22° 04′ 11.6″ N, 159° 18′ 50.5″ W), intertidal, 24 Feb. 1986.
Distribution
Endemic to the Hawaiian Islands (Bertsch & Johnson, 1982; Kay & Young, 1969).
External morphology (Fig. 17)
Commonly up to 200 mm. The notum is smooth. The body appears pyriform when the mantle is rolled and elongate-ovate when it is extended. The mantle extension is wider laterally but shorter on the head. The rhinophore sheath is slightly raised with a smooth edge. The rhinophores are slightly bent posteriorly with approximately 40 lamellae in large, mature animals. There are four to six multi-pinnate gill branches (usually five). The anus is elevated on a tubular papilla. The kidney pore is anterior to the anus on its right side. The oral tentacles are large, fleshy, and oval to elongate and crenate. The foot is narrower than the body.
Ontogeny, color, and variation (Fig. 17)
No photos or specimens were available of very young juveniles, but early transitional animals are translucent gray with a yellow marginal band, cloudy sub-dermal rosettes, and the beginning of red lateral patches. With growth, a submarginal red band develops. In mature animals, the background is typically dark red, and the sub-dermal rosettes are variably replaced with irregular patches of opaque white pigment (usually concentrated between the lateral patches). The dorsal margin of the innermost dorsal band is straight but not sharply defined. Rare animals may remain pale or be “frosted” with cream flecks when mature. The marginal yellow band is retained in mature animals, only rarely being replaced with white. The foot sole is lighter than the background color and has a yellow margin. The rhinophore lamellae lack white flecks and mature animals often have lateral striations. There does not appear to be any geographic variation within the archipelago.
As in H. sanguineus, during ontogeny, the mantle expands laterally and becomes rolled, the number of rhinophore lamellae increases and the gills become more elaborate. The notum of resting animals remains smooth.
Ontogeny, color, and variation in Hexabranchus aureomarginatus (Hawaii). Green circles indicate studied specimens and red online sources. A early transitional, B late transitional, C mature, Dmature, little white, E mature, extensive white, F mature, pale, G mature, frosted, H unrolled mantle, yellow margin, I unrolled mantle, white margin, J underside, K rhinophores, L gills, M oral tentacles, N egg mass, O–P mature in laboratory
Internal morphology (Fig. 18)
The buccal bulb is oval and slightly larger than the oral tube. The radula is broad and bilobed. The middle of the ribbon is devoid of teeth (Fig. 18A). The teeth are simple and hamate. The lateral teeth increase in length centrally and the outer teeth are smaller. (Fig. 18B). The innermost teeth are smaller or degenerate (Fig. 18C). The radular formulae is 32 × 57.0.57 (CASIZ 074,634). The jaws are armed with numerous simple, finger-like rodlets (Fig. 18D).
Reproductive system (Fig. 19)
Similar to Kay and Young (1969)’s description; however in the specimens examined by us, the female gland opened in a distinct genital atrium.
Hexabranchus aureomarginatus (CASIZ074271) reproductive system. A Photography, Breconstructed drawing. Abbreviations: amp, ampulla; bc, bursa copulatrix; dd, deferent duct; fmg, female gland; hd, hermaphroditic duct; p, penial bulb; pr, prostate; rs, receptaculum seminis; ud, uterine duct; v, vagina
Natural history and behavior
Hexabranchus aureomarginatus can be found in shallow water and tide pools and is most common at more exposed sites. It is a nocturnal feeder but appears to remain in the open by day more frequently than H. sandwichensis.
Remarks
The yellow margin is a distinctive character in H. aureomarginatus. Relative to the sympatric H. sandwichensis, the egg mass is lower, more loosely coiled, and usually lighter in color. The gills of H. aureomarginatus are typically held in a more erect position than in H. sandwichensis. The notum appears smooth in both resting and swimming mature animals and the genital papillae appear to have parallel margins in copulating pairs.
Hexabranchus morsomus (Marcus & Marcus, 1962) (Figs. 20, 21 and 22)
Caribranchus morsomus (Marcus & Marcus, 1962): Ortea et al. (2012: 24, Fig. 8D)
Distribution
Caribbean: British Virgin Islands (Marcus & Marcus, ), Puerto Rico (Marcus & Marcus, 1968), Guadeloupe (Ortea et al., 2012), Venezuela (Gutiérrez et al., 2015), Panama (Collin et al., 2005), Costa Rica, Honduras, Aruba, Puerto Rico, St. Marteen/St Martin, St. Lucia, Martinique, Antigua, Grenada, St. Vincent and the Grenadines, Trinidad & Tobago (Valdés et al., 2006).
Material examined
One specimen. CMPY000672, length 44 mm (preserved), Arrecife Caro, Arenas, Yucatán, Mexico, collected by Deneb Ortigosa, 26 May 2017.
External morphology (Fig. 20)
Commonly to 120 mm (with some reports to 200 mm). The body is oval. The examined specimen had a sub-pustulate to pustulate notum. Photographs of large mature, resting animals are usually strongly and evenly pustulate. The mantle is relatively wide, expanding and rolling on the sides and posteriorly, but not anteriorly. The mantle extends beyond the foot when expanded. The gills are compact and bushy with six tripinnate contractile (but non-retractable) gill branches distributed around an elevated anal papilla. The kidney pore is on the right side of the anal papilla. The gill branches are separated by thin tissue with two anterior branches clearly separated and four posterior branches arranged in pairs. The rhinophore is stocky and the club, in large mature animals, is relatively short with about 20 lamellae.
Ontogeny, color, and variation (Fig. 20)
No specimens or photos were available of very young juveniles. Nevertheless, Ortea et al. (2002) described an 8 mm specimen as white with a yellow edge, red rhinophores, and four white gill branches. Photographic material shows transitional animals to be white with variable red spotting on the pustules and a narrow red marginal line. With growth, the notum becomes mottled in red and cream. A broader, marginal red band with a straight and sharply defined inner margin develops on the dorsal surface. In large animals, the overall color darkens to red. The rhinophores are dark orange with white tips. Rhinophore lamellae may be sparsely flecked with white. The gills range from translucent red to translucent white with red lines on the rachis. The foot has a red margin.
Ontogeny, color, and variation in Hexabranchus morsomus. Green circles indicate studied specimens and red online sources. A early transitional, B mid-transitional, C late transitional, D mature, mottled, E mature, orange, F mature, red, G rhinophores, early transitional, H rhinophores, mature, I gills, light, J gills, dark, K oral tentacles, L swimming
As in other Hexabranchus spp., during ontogeny, the mantle expands laterally and becomes rolled, the number of rhinophore lamellae increases, the gills become more elaborate, and the notum of resting animals assumes the mature texture.
Internal morphology
Buccal mass (Fig. 21). The radula is similar to the ones described by Marcus and Marcus (1968) and Valdés (2002) (Fig. 21A–C). Nevertheless, only a few traces of a triangular rudimentary rachis could be seen (Fig. 21B). It is unclear if this is an artifact of the condition of the radula, an ontogenetic character, or a variable feature. Radular formulae 40 × 88.0(?)0.88 (CMPY000672). Jaw deprived of rodlets (Fig. 21D).
Reproductive system (Fig. 22)
As described by Marcus and Marcus (1962).
Natural history and behavior
Ortea et al. (2002) suggested that this species is an omnivore feeding on sponges, bryozoans, foraminiferans, and algae. According to them, the large oral tentacles are used to capture the high amount of food needed to supply the energy demands resulting from its large size and defensive swimming behavior. Other species of Hexabranchus have been found with different stomach contents apart from sponges (McDonald & Nybakken, 1997), but it is unclear whether or not they are accidental ingestions (Francis, 1980).
The examined specimen and online videos show that pustules on the notum tend to be less visible when the animal swims. The egg ribbon is relatively low, dull-red, and loosely coiled.
Remarks
This species is distinguishable from all other species of Hexabranchus by the absence of jaw rodlets and the presence of a vestigial triangular rachidian tooth. Mainly because of this, Ortea et al. (2002) doubted that H. morsomus could have a common ancestor with other Hexabranchus spp. and proposed the genus Caribranchus to accommodate it. Nevertheless, the presence of a vestigial rachidian tooth is not a strong diagnostic character as it is often hard to see and may vary at species level (e.g., Hoover et al., 2017). The type of labial cuticle is usually a clearer character, but it may vary within a genus (e.g., Neuhaus et al., 2021). Moreover, our phylogenetic analysis reveals that the genus Hexabranchus is monophyletic and H. morsomus is the sister species to all Indo-Pacific species. The closest related species to H. morsomus is H. aureomarginatus, with an elevated genetic distance of 13.26% (Table 3). They are easily distinguishable externally, but share a few similarities in the reproductive system, such as a female opening which appears to be visible in the genital atrium in both species, a loosely convoluted deferent duct (longer in H. aureomarginatus), and a large, curved prostate.
Hexabranchus giganteus Tibiriçá, Pola & Cervera, sp. nov. (Figs. 23, 24 and 25)
Zoobank Act. E9F31104-D3AD-409B-98AD-2614211B333A)
Material examined.
Holotype
MHNM.MOL.2022.0001 (sequenced), length 380 mm, Ponta do Ouro, “The Cake”, Mozambique (26° 50′ 22″ S, 32° 54′ 39″ E), 36 m, 12 April 2022.
Paratype
MB28-005009 (sequenced), length 110 mm, Ponta do Ouro, Atlantis, Mozambique (26° 50′ 58″ S, 32° 44′ 54″ E), 40 m, 16 July 2014.
Other material
MNCN:ADN 110938 (tissue), length 480 mm, Ponta do Atlantis, Mozambique (26° 50′ 58″ S, 32° 44′ 54″ E), 40 m, 16 April 2022; UL-YT1657 (dissected and sequenced), MNCN:ADN 110937 (tissue), length 430 mm, Nuarro, Nuarro Sacred Sands, Mozambique (14° 11′ 48″ S, 40° 59′ 18″ E), 04 Sep 2016; MNCN:ADN 110933 (tissue), length 420 mm, Ponta do Ouro, “The Cake”, Mozambique (26° 50′ 22″ S, 32° 54′ 39″ E), 42 mm, 23 July/2018.
Etymology
The specific name refers to the gigantic size of this species, one of the largest nudibranchs in the world.
Distribution
Western Pacific and Western Indian Ocean. Red Sea (Yonow, 2008), Djibouti, Yemen (Debelius, 1996), Mozambique (Tibiriçá et al., 2017), Seychelles, Madagascar (Debelius & Kuiter, 2007), Tanzania (Debelius, 1996), South Africa (pers. obs.), Hong Kong (Yonow, 2008), New Caledonia (Hervé, 2010), Indonesia (Tonozuka, 2003; Valdés, 2002), Papua New Guinea (Colin & Arneson, 1995; Debelius, 1996), Japan (Nakano, 2018) including Okinawa (Coleman, 2008). On-line sources: Oman, Philippines (iNaturalist), Emirates Arab (MedSlug), Mayotte (South-west Indian Ocean Seaslug site), Vanuatu (Underwater Australasia), and Fiji (Sea Slug Forum).
External morphology (Fig. 23)
Commonly up to 500 mm (with some reports to 700 mm in Madagascar). The notum of resting, mature animals is evenly pustulate (“quilted”). The body of mature animals is oval. The mantle is wide, expanding, and rolled on the sides and posteriorly (but not anteriorly). The foot extends slightly beyond the mantle. There are five to eight multi-pinnate gill branches separated in gill pockets. The anus is on an elevated papilla located in the center of the gill branches. The kidney pore is on the right side of the anus. The rhinophore clubs are elongate and slightly bent with approximately 80 lamellae (in large, mature individuals). The oral tentacles are large, fleshy, and elongated.
Ontogeny, color, and variation (Fig. 23)
No photos or specimens of very young juveniles were available but early transitional animals range from translucent gray to violet with orange rhinophores, orange gills with a cream rachis, a white marginal band, and a white rhinophore collar. Transitional animals develop diffuse pink spots on the notum. As the transition proceeds, the spots become sharply defined and darker pigment is deposited progressively outward from their boundaries filling the spaces between them (the process may not be fully complete until the animals reach 200–300 mm). As this happens, the notum becomes subpustulate and the rhinophores become yellow. In addition, reddish-pink patches appear on the underside of the mantle, on the mid-line of the “tail,” and on the lower portion of the rachis. Large animals may show thin, white, delicate lines that accentuate the pustulate appearance of the dorsum. In mature animals, the center of the notum is dark with lighter lozenges (corresponding to the diffuse pink spots of early transitional animals). The dorsal bands in mature animals are consistent in arrangement with a yellow marginal band, a dark submarginal band interrupted with elongate cream lozenges, and a yellow inner band. The background color is typically pink but varies from yellow to red. The gill rachis is cream grading to orange apically. The foot sole is yellow.
Ontogeny, color, and variation in Hexabranchus giganteus sp. nov. Green circles indicate studied specimens and red online sources. A late juvenile, B transitional, C late transitional, D mature, orange, E mature, red, F mature, dark red, G mature, yellow, H notum detail, I unrolled mantle, J underside, K rhinophores, late juvenile, L rhinophores, mature, M gills, N oral tentacles, O laying eggs, P egg mass
As in other Hexabranchus spp., during ontogeny, the mantle expands laterally and becomes rolled, the number of rhinophore lamellae increases, the gills become more elaborate, and the notum of resting animals assumes the mature texture.
Internal morphology (Fig. 24A)
The large, brown blood gland lies on top of the nerve ring. The light blue stomach is elongated and slightly wider than the intestine. The intestine passes between the reproductive system and digestive gland toward the elevated papillae anus. The gonad and digestive gland are cone-shaped, light brown, and bear light pink ducts.
Buccal mass (Fig. 24)
The radula (MNCN:ADN 110,933) is 23 mm long, broad, and bilobed (72 × 137.0.137). The anterior part of the radula is elevated (Fig. 24A). The lateral teeth are simple and hooked (Fig. 24B). The inner teeth are degenerate and triangular. The outer teeth are smaller (Fig. 24C). The jaw is smooth posteriorly with large and robust rodlets anteriorly (UL-YT1657, Fig. 24D).
Reproductive system (Fig. 25B–D)
Triaulic with the hermaphroditic duct leading to a very long, convoluted, pale pink ampulla. The ampulla passes between the female gland and the prostate gland, dividing into a short oviduct leading into the female gland mass and a deferent duct passing through the prostate portion. The prostate gland is granular, kidney-shaped, orange in color, stocky at its base, and narrowing toward the distal deferent duct. The distal deferent duct is thin and short leading to a muscular, thick portion. The deferent duct loops two to three times leading to the penial bulb that opens into a common atrium with the vagina. The vaginal duct curves once and then bifurcates, leading to the dorsal side of the bursa copulatrix and to the receptaculum seminis. The receptacle seminal is very long, convoluted, pale pink, and located dorsally to the bursa copulatrix. The bursa copulatrix is black, large, and oval. The short uterine duct emerges between the receptaculum seminis and the bursa copulatrix, entering the female gland. The female gland is half-oval, pale-pink, granular, and smaller than the bursa copulatrix.
Hexabranchus giganteus sp. nov. A Photography of internal anatomy of specimen UL-YT1657 with details of the reproductive system (ventral view above and dorsal view below). B Reconstructed drawing. Abbreviations: amp, ampulla; bc, bursa copulatrix; bg, blood gland; dd, deferent duct; dg, digestive gland; fmg, female gland; h, heart; hd, hermaphroditic duct; it, intestine; p, penial bulb; pr, prostate; reps, reproductive system; rs, receptaculum seminis; st, stomach, ud, uterine duct; v, vagina
Natural history and behavior. This is the largest species of the genus Hexabranchus. Its behavior is largely unknown; individuals have been seen crawling on the reef during day and night. Though it is probably feeding primarily at night we observed mating and egg-laying during the day. The notum of resting mature animals is regularly subpustulate but the subpustules are reduced in prominence during swimming. It is most common in deeper water (usually over 30 m), but there are occasional reports as shallow as 15 m in the Indian Ocean and 5 m in the Pacific. The egg mass is salmon, densely folded, and slightly disorganized. The gills are held in a more erect position than in H. lacer and the sides of the genital papillae appear tapered in copulating pairs.
The swimming pattern of this new species (under the name H. sanguineus) was studied using computational modelling and video by Zhou and Mittal (2017)
Can a Hexabranchus species still be undescribed?
Below we provide a commented list of all Hexabranchus species described so far including our reasons to allocate synonyms under each species here considered valid. This section provides a solid base to justify the introduction of a further species, as well as a tool to revise potential morphotypes not examined by us. Surprisingly, H. giganteus sp. nov. did not match any described Hexabranchus spp. This is likely due to its depth range (usually below 30 m) and to confusion in the literature. Overall, color pattern and notun texture associated with life stages were good indicators for species identification. Externally, H. giganteus sp. nov. differs from all other Hexabranchus species by its color combination, higher number of rhinophore lamellae, and outstanding size when adult. Internally, H. giganteus sp. nov. clearly differs by a much shorter deferent duct (Fig. 26).
Doris lacera Cuvier, 1804 (Fig. 26A)
Original illustrations of Hexabranchus species. A Doris lacer in Cuvier (1804). B Hexabranchus praetextus in Ehrenberg (1828). C Doris sanguinea in Rüppell and Leuckart (1830). D Doris marginata in Quoy and Gaimard (1832). E Doris flammulata in Quoy and Gaimard (1832). FHexabranchus sandwichensis in Gray (1850). G Doris sandwichiensis in Eydoux and Souleyet (1841). H Heptabranchus burnettii in Adams and Adams (1858). I Hexabranchus adamsii in Gray (1850). JDoris superba in Gould (1852). K Doris cardinalis in Gould (1852). L Doris sumptuosa in Gould (1852). M Hexabranchus pulchellus in Pease (1860). N Hexabranchus pellucidulus in Abraham (1876). O Hexabranchus suezensis in Abraham (1876). P Aethedoris indica in Abraham (1877). QHexabranchus orbiculares in Abraham (1877). R Hexabranchus faustus in Bergh (1878). S Albania formosa in Collingwood (1881). T Doris imperialis in Saville-Kent (1897). U Hexabranchus plicatus in Hägg (1901). V Hexabranchus marginatus var. moebii. in Bergh (1892). W Hexabranchus tinkeri in Ostergaard (1955). X Hexabranchus aureomarginatus in Ostergaard (1955)
Description notes
Described from preserved specimens; no notes on living animals; detailed internal morphology: size 90 mm; body relatively flattened; mantle slightly wider than a foot with a lacinate margin; large vesicles on notum; fleshy, slightly serrated oral tentacles; eight to ten gill leaves arranged separately around the anus; penis surrounded by a fleshy envelope; penial bulb colloidal; vas deferent long, thin, with several folds.
Own conclusion/opinion on its taxonomic status
Bergh (1900) investigated several preserved specimens of Hexabranchus spp. and concluded that 17 were synonyms of Hexabranchus lacer. Such material deposited in the Muséum National d’Histoire Naturelle (Paris), was later examined by Pruvot-Fol (1934), but she could not conclude whether the material represented one or more species and which species it represented. Baba (1988) stated that the lack of information about the coloration of the living organism and the potential distortion caused by preservation made the correct identification of H. lacer difficult and later authors preferred not to use this name (e.g., Edmunds (1971), Bergh (1905)). Since then, the name H. lacer has been largely ignored. Valdés (2002) examined the syntypes of Doris lacera and concluded that they belong to the genus Hexabranchus. Nevertheless, he declared Doris lacera “nomen oblitum” based on Article 23.9.1 of the International Code of Zoological Nomenclature (2000), which states that “if a senior synonym has not been used as a valid name after 1899 and its junior synonym has been used for the same species in at least 25 papers, published by at least 10 authors in the immediately preceding 50 years and encompassing a span not less than 10 years, the usage of the junior synonym must be maintained” However, Valdés (2002) missed Bergh (1900)’s reference and at least three others: Schauinsland (1899), Eliot (1904b) and Haas (1920). In addition, Valdés (2002) concluded that only one species was valid for the Indo-Pacific, and applying the principle of priority, he claimed Hexabranchus sanguineus to be nomem protectum. On the contrary, our results show that more than one species is present in the Indo-Pacific and that H. lacer and H. sanguineus are distinct species.
Specifically, at least three species are present in the Indonesian region: H. lacer, H. sanguineus and H. giganteus. Despite lacking color details, Cuvier’s (1804) description is sufficiently detailed to confidently attribute this name to a Hexabranchus species widely distributed in the Indo-Pacific. This decision is based on the integration of location, internal morphology, and general appearance. Cuvier’s (1804) drawing clearly shows a very long and coiled penis and deferent duct, excluding the possibility of it being H. sanguineus or H. giganteus. Furthermore, Doris lacera was described presenting nine to ten gill branches, while H. sanguineus typically has six. Cuvier’s (1804) drawing shows incongruent vesicles on the dorsum, but we noted that vesicles may or may not be formed (in multiple species) due to preservation. We find it unlikely that H. lacer represents H. giganteus sp. nov., as the latter is mostly found at depths greater than 30 m and a specimen measuring 90 mm (preserved) would be immature. As a result, we resurrect H. lacer.
Hexabranchus praetextus Ehrenberg, 1828 (Fig. 26B)
Description notes
short description based on external features: flattened body; foot narrower than mantle; red notum; white margin; six gill branches arranged around anus in separate gill pockets; oral tentacles serrated.
Own conclusion/opinion on its taxonomic status
There is no doubt that H. praetextus and H. sanguineus represent the same species. H. praetextus and H. lacer were declared nomen oblitum by Valdés (2002) based on ICZN Art. 23.2. The International Code of Zoological Nomenclature (2000) establishes that such action can be done when two conditions are met: (1) Art. 23.9.1.1, the senior synonym has not been used as valid after 1899, and (2) Art. 23.9.1.2, the junior synonym has been used in at least 25 publications by at least 10 authors in the immediately preceding 50 years and encompassing a span of not less than 10 years. Despite H. sanguineus being extensively used in the literature by almost all authors, H. praetextus was cited as valid in at least one publication after 1899 (see Vayssière, 1912). Therefore, contrary to what was stated by Valdés (2002), condition 23.9.1.1 was not met. Nevertheless, according to the code, “if action taken under Article 23.9.2 is found later to have been taken in error in those conditions 23.9.1.1 and 23.9.1.2 were not met, the case is to be referred to the Commission. Prevailing usage must be maintained [Art. 82] until the Commission has made a ruling.”
Taking into account article 23.9.3 which says “If the conditions of 23.9.1 are not met but nevertheless an author considers that the use of the older synonym or homonym would threaten stability or universality or cause confusion, and so wishes to maintain use of the younger synonym or homonym, he or she must refer the matter to the Commission for a ruling under the plenary power [Art. 81],” we highly recommend that H. sanguineus remains the valid name. Our recommendation is based on the fact that an action of erroneous reversal of precedence would add even more confusion to the literature. Additionally, H. sanguineus is a popular name among the scientific and non-scientific community and has been used in all recent field guides and manuscripts.
Doris sanguinea Rüppell and Leuckart, 1830 (Fig. 26C)
Description notes
Description based on three specimens; no internal features included: approximate size 150 mm; mantle margin extended, except anteriorly; blood-red notum; white band surrounding mantle; upper rhinophores darker; often, six gill leaves; gill leaves whitish-rose, quite far apart; smaller specimen with five gill leaves, one appearing to be fused; gills contractile, non-retractable.
Doris marginata Quoy and Gaimard, 1832 (Fig. 26D)
Description notes
Only external morphology: size 200 mm; body red, oval; mantle wide, margin undulating all around except on head; gill branches arranged around anus and forming seven tufts; oral tentacles oval and crenate; specimen observed swimming violently.
Own conclusion/opinion on its taxonomic status
This species was by most authors regarded as a junior synonym of H. sanguineus. However, Yonow (2001) suggested that H. sanguineus was endemic to the Red Sea and this species should be considered valid. The species epithet “marginatus” was the second available name for the widely distributed Indo-Pacific “Hexabranchus lacer.” Because the description of “H. lacer” did not include coloration and present distinct pustules, several authors overlooked that name and applied “marginatus” for that species (Baba, 1936; Kay & Young, 1969; Risbec, 1928; Yonow, 2001). Nevertheless, here we show that “H. lacer” should have priority over “marginatus.” The illustration shows that this species is a morphotype of H. lacer differing from H. giganteus sp. nov. of similar size by the lack of pink blotches, a dark red submarginal band lined in white and dorsum texture.
Doris flammulata Quoy and Gaimard, 1832 (Fig. 26E)
Description notes
Only external morphology: size 100 mm; mantle soft, thin, edge lacinate; generally red-brown; “gnawed” background with small yellow dots around the main dorsal region and a series of yellowish flames with red dots; mantle margin cherry red, edges white; gill branches vary from seven to eight; underside colored as notum, but more yellow.
Own conclusion/opinion on its taxonomic status
Our study reveals that this morphotype is one of the many variations of H. lacer. This species could not be H. giganteus sp. nov. because individuals of similar sizes of H. giganteus sp. nov. bear whitish dorsum with yellow edge and pinkish blotches (not dots).
Hexabranchus sandwichensis Gray, 1850 (Fig. 26F)
Description notes
No description, only figure. Latter Eyodoux and Soulet (1852) provided external morphology: size 140 mm; smooth notum bluish-white trending to purple in the central region and marked by irregular purple spots; whitish band surrounding the foot underneath the mantle; mantle expanded around the body; foot yellowish (Fig. 26G).
Own conclusion/opinion on its taxonomic status
In WoRMS (MolluscaBase eds., 2023c), Russel (1971), and Valdés (2002), H. sandwichensisappears as authored by Gray, 1850. In contradiction, Edmunds (1971) and McDonald (2021) suggested Eydoux and Souleyet (1852) as the correct authors. The nomenclatural history dealing with “Gray, 1850” is confusing and often ignored (see Valdés & Fahey, 2006). Gray’s (1850) work is a compilation of illustrations made by Maria Emma Smith (M. E.) Gray for her husband’s (John Edward Gray) personal use. Mrs. M. E. Gray traced hundreds of mollusk figures from several authors. Regretfully, it appears that in a few cases the drawings were introduced prior to the original drawing creating some problems (Valdés & Fahey, 2006) as under Art. 12.2.7 these names should be considered available. In the case of H. sandwichensis, it is possible that this species was first introduced by Gray’s illustration in 1850 and later described by Eydoux and Souleyet in 1852. Conversely, Adams (1848), when he described H. burnettii, compared this species with “Doris sandwichiensis” in “Voyage de la Bonite,” an expedition that took place between 1836 and 1837. Sherborn (1901) cites that the correct dates of the plates from such publication are unknown and tentatively refer to 1846–1849. Therefore, it would be possible that Eydoux and Souleyet published the illustration prior to Gray (1850) but regrettably the dates are unconfirmed and the name “Doris sandwichienne” was written in French and not latinize. Thus, based on our results and review Hexabranchus sandwichensis Gray, 1850 is a valid species.
Heptabranchus burnettii (Adams, 1848) (Fig. 26H)
Description notes
Brief description based on external morphology: seven gill leaves in individual pockets forming a semi-circle around a tubular anal papilla; wide mantle extending beyond the foot.
Own conclusion/opinion on its taxonomic status
This species was described under the genus “Heptabranchus” by bearing seven gill leaves. No illustration was provided with the descriptive notes but, in 1858, a drawing appears in Adams and Adams (1858, pg. 59) as “Hexabranchus Burnettii. Original. A. A.” (Fig. 26H). Adams (1848) suggested that “Doris sandwichienne” was similar to H. burnettii; however “Doris sandwichienne” shows a wider mantle and seven (instead of eight) gill leaves arranged in a semi-circle. He mentions similarities with H. praetextus but the latter differed from H. burnettii by bearing six gill leaves in distinct pockets. Bergh (1880) considered H. burnettii(together with H. pellucidulus and H. adamsii) “unsafe” descriptions, perhaps because he did not examine the illustrations. Valdés (2002) could not find the holotype of this species but synonymized it as H. sanguineus. Despite the doubtful description, the illustration confirms the generic placement as a Hexabranchus. In fact, the illustration is very similar to the morphotype described as Doris flammulata, and almost identical to a juvenile specimen of H. lacer examined by us. The unusual semi-circular gill arrangement is likely an artifact of body position and preservation. Therefore, we consider H. burnettii a junior synonym of H. lacer(instead of H. sanguineus).
Hexabranchus adamsii Gray, 1850 (Fig. 26I)
Description notes
Text description not provided, only figure.
Own conclusion/opinion on its taxonomic status
Gray’s (1850) trace shows a typical juvenile of H. lacer with distinctive spots surrounding dots. H. sandwichensis also presents a similar pattern but such species is restricted to Hawaii. Therefore, there is no doubt that H. adamsii Gray (1850) is a junior synonym of H. lacer.
Doris superba Gould, 1852 (Fig. 26J)
Description notes
Specimen described based on external features. Size 140 mm; mantle wide on the sides, emarginate anteriorly; background color intense red with minute yellow dots; six double-gills; lobate oral tentacles.
Own conclusion/opinion on its taxonomic status
the author states that this species is similar to “Doris sandwichiensis” but the red is more intense and concentrated, the mantle does not extend onto the anterior side of the body, and the shape of the oral tentacles is more lobate. Furthermore, Gould (1852) adds that this species is almost identical to Doris flammulata except for the shape of the oral tentacles and the number of gill branches. According to our findings, the minute, evenly distributed white dots on a dark background suggest that this name refers to the dark morphotype of H. lacer.Again, the coloration pattern associated with the size excludes the possibility of this species being H. giganteus sp. nov.
Doris cardinalis Gould, 1852 (Fig. 26K)
Description notes
Description based on external morphology only: size 150 mm; body large, oval when extended; color mottled yellowish and red, somewhat violaceous in the transverse depressions; a few purple blotches on the notum; mantle extension wider on sides, narrower posteriorly, differentiated anteriorly; mantle extension deep red, margin thin, white; rhinophores red; six rose-red dendritic gill leaves; two-lobed oral tentacles.
Own conclusion/opinion on its taxonomic status
Gould (1852) stated that this species from Hawaii is an intermediate form between Doris sumptuosa and D. superba and quite similar to “D. sandwichiensis” but with distinct gill leaves, oral tentacles, and color underneath the mantle. Based on the illustration, we believe that this species is probably H. sandwichensis. The mottling in the center of the notum, lateral to the depression, and on the underside are typical for this species. The diffuse margins of the depression are also consistent. The penciled dark spots on the notum resemble the scattered darker patches that are often present in H. sandwichensis. The marginal white line is too narrow for H. aureomarginatus, whereas a narrow white line is often noted in H. sandwichensis. The well-defined red band underneath the margin is typical of H. sandwichensis. Therefore, we consider Hexabranchus cardinalis (Gould, 1852), a junior synonym of H. sandwichensis.
Doris sumptuosa Gould, 1852 (Fig. 26L)
Description notes
Only external morphology provided: size 270 mm; four transverse constrictions on notum; background cherry-red with purple and yellow dots on the notum and yellow blotches at constrictions; rhinophores rose.
Own conclusion/opinion on its taxonomic status
The author mentions that the species is similar to Doris marginata but that the oral tentacles are ovate, delicate, and crenate. He also states that there are more gill leaves (around twelve). According to our findings, H. lacer is the valid name for D. marginata and the number of gill leaves is variable in that species. Additionally, a close look reveals a mottled central notum and undulating margins on the lateral red bands. Thus, no substantial differences can be seen between H. sumptuosa and H. lacer. We consider D. sumptuosa Gould (1852), a junior synonym of H. lacer.
Doris gloriosa Kelaart (1858)
Description notes
Description of external morphology only; no illustration provided: size 70 mm; seven to eight gills arranged around the anus in separate holes; notum pink, mottled and covered in red, yellow, and white dots; mantle margin white, sub-margin red; interior to this, a whitish band followed by red with “internal club-shaped prolongations” (page 92); second specimen with a cherry red edge (instead of white).
Own conclusion/opinion on its taxonomic status
The author noted the swimming behavior and that the appearances of the animals change when rolling the mantle making them look like different species. Keelaart (1858) thought this species was likely Doris marginata. Nevertheless, he concluded that D. marginata’s description was a bit vague and could be applied to several other species. Therefore, he decided to provide a new specific name. The color description clearly reveals that this species represents a transitional morphotype of H. lacer.
Hexabranchus pulchellus Pease, 1860 (Fig. 26M)
Description notes
Brief description based on external morphology: seven gill leaves surrounding the anus, each retractile into separate cavities; undulating margin; notum yellowish, covered in red dots; mantle edge white with red dots; gill leaves pale, lined in red.
Own conclusion/opinion on its taxonomic status
From our analyses and type location (Hawaii), it is evident that this species is a juvenile and a junior synonym of H. sandwichensis.
Hexabranchus pellucidulus Abraham, 1876 (Fig. 26N)
Description notes
Described from a preserved specimen; external morphology and brief radula description: size 27 mm (preserved); subpustulate on the back; lateral mantle not very wide when compared to others; small bipinnate gill leaves relatively close to each other; anal papilla slightly raised; less gelatinous texture anterior to the rhinophores; oral tentacles fleshy, crenate; color transparent white, yellowish; radula broad and bilobed with numerous hamate teeth.
Own conclusion/opinion on its taxonomic status
The author distinguished this species (together with H. lacer) from H. gloriosus by the dorsal sub-pustules. He further differentiated it from H. lacer by the latter having a more fimbriate mantle edge and conical pustules. However, the specimen was preserved, and we noticed that this characteristic can be an artifact of preservation. Unfortunately, since the type locality is unknown, and the description does not provide sufficient detail to accurately identify the species, H. pellucidulus is here considered a nomen dubium.
Hexabranchus suezensis Abraham, 1876 (Fig. 26O)
Description notes
Described from a preserved specimen; only external morphology provided: size 100 mm (preserved); body elliptical, smooth; mantle expanded around the whole body, wider posteriorly with a fimbriate edge; six non-retractile, small multi-pinnate gill leaves; oral tentacles fleshy, oval and multilobate; color light brownish in spirits.
Own conclusion/opinion on its taxonomic status
Abraham (1876) stated that this species is differentiated from H. praetextus and H. sanguineus by a more elongated shape and a more depressed body. Nevertheless, these characteristics may change with preservation and body position. The type location (Red Sea), smooth notum, and the well-defined six gill branches suggest that H. suezensis is indeed H. sanguineus. Hexabranchus suezensis was synonymized as H. sanguineus by Thompson (1972), confirmed by Valdés (2002) with which we concur.
Hexabranchus souleyeti Bergh, 1877
Description notes
Not provided.
Own conclusion/opinion on its taxonomic status
It seems that Bergh (1877) used this name to refer to the species described by Souleyeti as D. sandwichiensis. Thus, it is a replacement name for H. sandwichiensis (Eydoux & Souleyet, 1852), which was preoccupied by H. sandwichensis (Gray, 1850).
Aethedoris indica Abraham, 1877 (Fig. 26P)
Description notes
Brief description based on a colorful illustration: wide mantle; large, bilobed oral tentacles with crenate edges; notum mottled, red.
Own conclusion/opinion on its taxonomic status
Abraham (1877) only examined a figure published in Alder and Hancock (1864) as an undescribed species, which got his attention because of the “expansion of the bilobed head” (page 237). Alder and Hancock (1864) included in their publication illustrations made by native Hindu artists during an expedition led by Eliot from 1853 to 1854 to Waltair, India. Regrettably, no text description was provided, and specimens were not collected. Since the illustration (with a certain artistic license) shows a mottled notum and undulating lateral band, we suggest that this species is likely a junior synonym of H. lacer (instead of H. sanguineus).
Hexabranchus orbicularis Abraham, 1877 (Fig. 26Q)
Description notes
External features observed on a freshly preserved specimen: size 72 mm; body depressed, oval; mantle expanded around the body except anteriorly; numerous gill leaves grouped in eight tufts; gill leaves white, red lined; notum purple-red, mottled with white blotches; mantle lateral extension dark with radiating streaks of red, white blotches and a white edge; red rhinophores; gill branches white, lined in red; underside of mantle mottled; oral tentacles fleshy, oval with a crenate edge.
Own conclusion/opinion on its taxonomic status
The color description was based on a recently preserved specimen and may not be totally accurate. Nevertheless, the red radiating streaks at the margin exclude the possibility of being H. giganteus sp. nov. The general coloration together with the number of gill leaves suggest that this species is likely a junior synonym of H. lacer (instead of H. sanguineus).
Hexabranchus aneiteumensis Abraham, 1877
Description notes
Brief description of the external morphology of a preserved specimen; no illustration provided: body oval, depressed; mantle expansion wider on sides and posteriorly, smooth with a fimbriate edge; seven gill tufts arranged around the anus in different cavities; oral tentacles fleshy, multi-lobed; color in spirits mottled red and white on notum, reticulated and spotted with red on underside; foot sole red.
Own conclusion/opinion on its taxonomic status
The species was described from a preserved specimen and the color description is lacking in detail. In general, the mottled coloration and Western Pacific location would suggest that this species is a possible junior synonym of H. lacer. The depressed body and red foot sole allow us to discard H. giganteus sp. nov.; however, it does not allow us to fully discard H. sanguineus. Therefore, we consider H. aneiteumensis as nomen dubium.
Hexabranchus mauritiensis Abraham, 1877
Description notes
External morphology and brief radula description with no illustration; based on preserved specimens: maximum size 75 mm; body elliptical, moderately depressed, smooth; mantle expansion fimbriate, except anteriorly; seven to eight non-retractile tripinnate gill leaves arranged in a circle around the anus; oral tentacles fleshy, leaf-like with a crenate edge; color in alcohol grayish-white; radula with 30 rows of numerous hamate teeth; teeth less curved than in H. aneiteumensis.
Own conclusion/opinion on its taxonomic status
The number of gill branches and location suggest this species is H. lacer. Nevertheless, the description does not provide sufficient details to determine which species the author was referring to. Therefore, this name should be considered a nomen dubium.
Hexabranchus anaiteus Bergh, 1878
Description notes
Description based on internal and external morphology of three preserved specimens; illustration only shows radula and jaw. Maximum size 90 mm; preserved color red, mottled in purple on the notum; mottled underside; mantle margin yellow; foot sole red; body shape “as usual”; six to nine, commonly six, tripinnate gill tufts; anal papilla elevated; kidney orifice on the right side; oval oral tentacles; radula very large and strong with 47 rows of simple, hook-shaped curved teeth; thin vas deferent leading to a spiral penis.
Own conclusion/opinion on its taxonomic status
Bergh (1900) synonymized this species as H. lacer and later Thompson (1972) changed it to the synonym of H. sanguineus. By the location, mottled color, and description of the reproductive system, this species is likely a junior synonym H. lacer. The coloration of a relatively small specimen and the spiral penis excludes the possibility of being H. giganteussp. nov.
Hexabranchus faustus Bergh, 1878 (Fig. 26R)
Description notes
Description based on an illustration of living specimens and three preserved specimens; internal and external morphological details provided; notes from a living specimen (and its illustration) provided by Semper: living animal 80 mm; preserved specimens from 65 to 95 mm: body oval, depressed, slightly wider posteriorly; notum pale red with whitish marks and darker dots; mantle expansion with dark purple spots on its inner sides followed by a white band and dark reddish-gray marginal band around the mantle edge (except anteriorly); rhinophores and gills colored like the notum except for yellow tips on the gill tufts; rhinophores slightly arched backward with 50 lamellae; text describes six triple pinnate gill branches arranged in six tufts but the illustration shows seven; radula with 48 rows of teeth, 15 rows more developed; curved teeth; reproductive system enclosed by connective tissue; penis almost cylindrical forming about five spirals.
Own conclusion/opinion on its taxonomic status
Geographic distribution, external morphology (in particular the distinct white/red mantle marginal band), and the penis forming spirals clearly confirm the identification as H. lacer. Therefore, H. faustus is here transferred from a junior synonym of H. sanguineus to a junior synonym of H. lacer.
Hexabranchus faustus var. Bergh (1878)
Description notes
Description based on the preserved specimen; external and internal morphology provided; illustrations of jaw and radula details only: size 45 mm (preserved); shape oval, slightly wider posteriorly; mantle fairly broad; oral tentacles thick, with a wavy edge; rhinophores with 40–45 lamellae; six gill tufts; color bright yellow on sides and foot (preserved); radula typical with 22 hamate tooth rows; reproductive system not developed.
Own conclusion/opinion on its taxonomic status
This variation was described from a single individual collected by Semper, it differs from H. faustus, above, only in minor details such as a broader mantle and a different radular formula. These differences are not sufficient to separate the species.
Hexabranchus petersi Bergh, 1878
Description notes
Description based on preserved specimens with notes on living animals, external morphology, and internal morphology; illustration of anal opening, kidney pore, and parts of the reproductive system: in life, a red mantle with white edge; size from 32 to 80 mm (preserved); flattened body; broad mantle; rhinophores with 45–50 lamellae; number of gill tufts vary from 6 to 8, commonly five; anal papilla moderately elevated; thick, oval, crenate oral tentacles; teeth hamate; radular formulae 22 × 66.0.66.
Own conclusion/opinion on its taxonomic status
Bergh (1878) states that this species has a less flattened profile than H. praetextus, but the color and, in fact, most of the description are very similar. The shape of Hexabranchus spp. varies tremendously according to the preservation process. There is no reason to distinguish H. petersi from H. praetextus and H. sanguineus. Therefore, this species should remain a junior synonym of H. sanguineus.
Hexabranchus notatus Bergh, 1878
Description notes
Description based on one juvenile specimen, preserved; illustrations of morphological parts only (jaw and radula details, anal papilla, kidney pore, and salivary gland): size 25 mm; body slightly more elongated and elevated posteriorly compared to others; oral tentacles fleshy and serrated; color of preserved specimen light gray; mantle expansion, foot and gill tufts darker; rhinophores green-olive; jaw rodlets rounded, irregular; radular formulae 34 × 57.0.57; reproductive system not developed.
Own conclusion/opinion on its taxonomic status
There are insufficient details to assign this name to a species. This name should be considered as nomen dubium.
Albania formosa Collingwood, 1881 (Fig. 26S)
Description notes
Description of a single specimen, observed alive; only external morphology provided: length 50 mm; mantle extended on the side, able to roll; rhinophores thick, without sheath, non-retractile; seven gill tufts separated in individual cavities within a common, delicate ring; background color pale red, darker on the central notum; red “forms” on the side with minute whitish dots forming shapes; oral tentacle crenated; foot extending beyond mantle.
Own conclusion/opinion on its taxonomic status
the genus Albania Collingwood (1881) was created to accommodate species closely related to the genus Hexabranchus but with seven retractile gill tufts. The author includes a note of its swimming behavior as typical of Hexabranchus spp. No specimens handled by us fully retract their gills, but they were able to contract them when disturbed and (in a juvenile) within the major cavity. The apparent odd gill arrangement is typical of a juvenile Hexabranchus. This species matches H. sanguineus from the West Pacific in the illustrated pattern. The inner margin of the lateral red band is strait rather than undulating, the lateral patches are truncated medially and elongated laterally and there are several disjunct, round patches between them. Together, these markings are very different from typical H. lacer. The pencil stippling on the notum corresponds to the dense patches of minute white flecks that are also characteristic of H. sanguineus, West Pacific morphotype. The rhinophores appear slenderer and the gills more sparsely branched than what is commonly seen in H. lacer. Seven gill branches are within the range of variation for H. sanguineus and H. lacer.
The overall appearance suggests that this species is a junior synonym of H. sanguineus.
Hexabranchus imperialis (Saville-Kent, 1897) (Fig. 26T)
Description notes
Very brief text description of a living specimen with illustration: size approximately 250 mm; body oval, depressed; color predominantly red with a white-decorated marginal band.
Own conclusion/opinion on its taxonomic status
The description lacks details of both internal and external morphology. The general shape, coloration, and illustration provided resemble the genus Hexabranchus, but the rhinophores and gill branches are inconsistent. Nevertheless, it was transferred to the genus Hexabranchusby Basedow and Hedlet (1905). The wide marginal band with radiating folds can occur in H. lacer. The laterally placed, round dark blotches are suggestive of the disjunct round patches common in H. sanguineus. The lateral band margins and lateral patches seem ambiguous. This species cannot be assigned conclusively to either species. So, it should be considered a nomen dubium.
Hexabranchus plicatus Hägg, 1901 (Fig. 26U)
Description notes
Description of external morphology with color notes on a living specimen: size 100 mm; body oval; mantle expanded around the body; mantle edge undulating, except anteriorly; body dark red with a white marginal band; six large gill leaves separated equally; kidney-shaped oral tentacles, crenate; lamellate rhinophores, bent backward; anus elevated; anterior excretory orifice on right side, posteriorly; on left side a small papilla of unknown function.
Own conclusion/opinion on its taxonomic status
By the external description and location, there is no doubt that this species should remain as a junior synonym of H. sanguineus.
Hexabranchus digitatus Eliot (1903)
Description notes
Species described from a badly preserved specimen with no illustration and no details of coloration or characteristics of the living specimen: reproductive system, nervous system, and buccal mass cited as “usual” (pg. 903); size 78 mm; mantle extremely wide and wrinkled; oral tentacles deeply crenate; three digitate mantle projections on head; gill branches too badly conserved to enable counting.
Own conclusion/opinion on its taxonomic status
The description of this species is too poor for any conclusion so it should be considered a nomen dubium.
Hexabranchus marginatus var. moebii Eliot (1904a, b) (Fig. 26V)
Description notes
Description of this variety was made by Eliot (1904b) based on a systematic review performed by Bergh (1892): notum varies in color from light red to whitish, frequently mottled; distinct red and white bands surround the mantle from which smaller and larger areas alternately run inwards; six to eight gill leaves, often separated by a little tissue and appearing higher in number.
Own conclusion/opinion on its taxonomic status
Bergh (1892) re-describe H. marginatus including two variations. Eliot (1904b) thought that one of the variations was distinct due to the peculiar red/white band, referring to this as var. ‘moebii’. Despite the color differences, Eliot (1904b) did not find any structural differences between these varieties, H. faustus and H. marginatus. Our results show that they are all variations of H. lacer: a typical light morphotype with a mottled notum, a darker morphotype in which dark pigment obscures the mottling/lateral markings and a juvenile. Online pictures show the lighter and darker morphotypes mating (e.g., https://www.istockphoto.com/es/foto/bailarina-española-gm1328642884-412631041).
Hexabranchus punctatus Bergh (1905)
Description notes
Description based on preserved specimen; external and oral mass morphology provided; only a tooth illustrated: size 20 mm; mantle narrower than usual, not undulating; six gill branches; rhinophores bent strongly backwards; armed jaws; radular formulae 38 × 37.0.37.
Own conclusion/opinion on its taxonomic status
Bergh (1905) pointed out that the general appearance was “as usual” but that the background color was yellowish with scattered black spots on the notum and underneath the mantle. He differentiated it from other species mainly because of a non-undulating and narrower posterior part of the body. Eliot (1908) thought the description was lacking and suggested it might be the same as H. adamsii Eliot, 1905. By the size, location, and description, we believe that H. adamsii and H. punctatus are, respectively, early juvenile and midle-late juvenile forms of H. lacer.
Hexabranchus tinkeri Ostergaard, 1955 (Fig. 26W)
Description notes
Description based on several specimens: up to 230 mm in length; notum mottled in bluish white, reddish, and yellowish; a light bluish area and carmine spots surround the central region of the notum; six to eight large gills; expansion of the lateral mantle similar in color to the notum except for a sub-marginal red band and marginal pale carmine band; rhinophore sheaths low, white; oral tentacles scalloped.
Own conclusion/opinion on its taxonomic status
Ostergaard (1955) mentioned that he found little variation except in three specimens collected later in which the lateral mantle expansion was wider with a deep carmine submarginal band. He distinguished this species from H. sandwichensis by its coloration and gill arrangement. Ostergaard (1955) believed that H. sandwichensis was a rare species because in 30 years he did not find any animals like it. Furthermore, the author distinguished this species from H. cardinalis by the bilobed oral tentacles. Our analysis reveals that this species is, in fact, the adult form of H. sandwichensis.
Hexabranchus aureomarginatus Ostergaard (1955) (Fig. 26X)
Description notes
No details of the internal morphology provided: from 76 mm (holotype) up to 170 mm; body depressed; notum bright orange to orange with variable white marks.; four to six gill branches, relatively small; rhinophore sheath elevated, reddish, orange, and white; oral tentacles scalloped.
Own conclusion/opinion on its taxonomic status
Color variation was noted mainly on the foot sole which ranged from yellowish to deep carmine, as well as in the overall color intensity. The yellow margin appears consistent in all variations. Our analysis confirms its close relationship to H. sanguineus and that it is a separate and valid species endemic to Hawaii.
Hexabranchus morsomus Marcus & Marcus, 1962
Description notes
Description based on preserved material with a short note on the living specimen, internal morphology, and external morphology; only radula and reproductive system illustrated: size 30 mm (preserved); notum bright red and cream (alive); dorso-ventral swimming behavior; body smooth, broad (preserved); large crenate oral tentacles; six tripinnate gill leaves around an elevated anal papilla; reproductive system with two genital openings; radula formulae 40 × 90.1.90; rachis rudimentary and triangular.
Own conclusion/opinion on its taxonomic status
Thompson (1972) stated that the geographic distribution and radula details were the only relevant distinguishing characteristics between this species and Indo-Pacific Hexabranchusspp. Despite this comment, he did not explicitly define his position regarding the taxonomic status of H. morsomus which remained uncertain until Valdés (2002) confirmed it as a valid species (which is further verified by our molecular analysis).
Discussion
Biogeography
The genus Hexabranchus is a typical genus from tropical and sub-tropical regions with its highest diversity found in the Indo-Pacific. The Hawaiian Archipelago is well known for its unique marine diversity with over 10% of the species being endemic (Briggs, 1999). This is particularly evident in this genus as two out of six Hexabranchus species are exclusively found there.
Hexabranchus species occur in the Caribbean (but, nowhere else in the Atlantic) and from Hawaii to the western Indian Ocean in the Pacific but are absent in the Eastern Pacific. Geographic dispersion and evolutionary history are a complex subject, beyond the scope of this study. Nevertheless, in this paper, we confirm the monophyly of the genus Hexabranchusand validated that both speciation hypotheses proposed by Valdés (2002) could be valid: (1) a split of an ancestor due the closure of the Isthmus of Panama, followed by speciation and the extinction of the genus in the Eastern Pacific; or (2) an origin in either the Indo-Pacific or the Caribbean followed by colonization to the other region prior the closure of the east–west communication. A third hypothesis would be a more recent colonization from the Indian Ocean to the Caribbean, likely before the formation of the Benguela cold-water barrier about 2 million ago, followed by speciation.
Our phylogenic analysis suggests that H. morsomus is sister to all other species of the genus. Both the p-distance and theCOI haplotypes revealed that H. morsomus is genetically closest to H. aureomarginatus from Hawaii. These two facts associated with massive extinctions of marine invertebrates due to the closure of the Panamanian Seaway (Leigh et al., 2014), suggest that the first hypothesis is the most plausible. In fact, it is likely that the closure of the Panama Seaway affected many other marine heterobranchs as this atypical distribution is seen in other genera such as Mannesia Zamora-Silva and Malaquias (2017), Mariaglaja Zamora-Silva and Malaquias (2017), Nakamigawaia Kuroda and Habe (1961) (Zamora-Silva & Malaquias, 2018) and Thuridilla Bergh (1872) (Martín-Hervás et al., 2021).
Additionally, in all three widely distributed species of Pacific Hexabranchus appears a disjunct (with the disjunction in H. lacer being somewhat speculative). Such a pattern is less common than an anti-tropical disjunction and may be related to extinction events caused by the interruption of east–west dispersal (Briggs, 1999). Alternatively, this apparent disjunction could be an artifact of uneven sampling. Nevertheless, the morphological variations found in H. sanguineus and the haplotype network analysis suggest that the east–west barrier indeed has reduced gene flow in this species.
Taxonomic placement
Ortea et al. (2002) erected the genus Caribranchus to accommodate H. morsomus as they hypothesized that H. morsomus and H. sanguineus had a different ancestor. These authors believed that H. morsomus was more likely related to the genus Aphelodoris, as they share similar geographic distributions and morphological characteristics. Nevertheless, our phylogenetic analysis reveals that this is not the case and that H. morsomus is the sister species of all other Hexabranchus spp. In addition, Ortea et al. (2002) pointed out that some morphological characteristics like the radula and jaws do not fit the diagnostic characters of the genus Hexabranchus. Yet, the combination Caribranchus morsomus has been largely ignored in the literature. Our phylogenetic analysis proves the monophyly of the genus Hexabranchus. Even though the buccal apparatus is distinct in H. morsomus, several of its characters are exclusive to Hexabranchus and can be used for diagnosis. Therefore, we agree with Valdés et al. (2006), who considered the monospecific genus Caribranchus unnecessary. Thus, we provide an amended diagnosis (under systematics) and formally place CaribranchusOrtea et al. (2003) as a junior synonym of Hexabranchus.
The higher taxonomic relationships of the family Hexabranchidae Bergh (1891) remain unresolved. Recent studies applying molecular techniques show that the family is closely related to Cadlinellidae Odhner (1934) but the sister relationship is poorly resolved. For example, Hallas et al. (2017) noted that in their phylogeny of Doridina, the family Hexabranchidae was recovered as the sister of Cadlinella ornatissima (Risbec, 1928), but mentioned that the significance of its support was unclear and depended on how the variable regions in the alignments were cured. Recently, Korshunova et al. (2020) proposed that Cadlinellidae Odhner (1934) and Korshunova et al. (2020) were sister to Hexabranchidae. However, neither in our phylogeny nor in the phylogeny provided by them was this relationship strongly supported (PP = 0.94; BS = 50, Korshunova et al., 2020).
Ontogeny and color patterns
Understanding the ontogenetic changes in nudibranchs may help to solve some of the enigmatic evolutionary paradigms such as gill formation (Korshunova et al., 2017; Martynov, 2011). The gill arrangement of the genus Hexabranchus is of particular interest as dorid nudibranchs have been traditionally divided into “cryptobranchs” (species able to fully retract their gills into a gill cavity) versus “phanerobranchs” (species without a gill cavity and unable to retract their gills). The phylogenetic validity of such a division is questionable and heavily debated. Recently, Korshunova et al. (2020) inferred that in early juvenile H. lacer (cited as H. sanguineus), the gill is arranged within a cavity-like depression and that they separate with growth. Nevertheless, a direct examination of several young specimens reveals that such a statement is only partially true. Gill branches in H. lacer and H. sandwichensis are always separated in individual pockets. However, when they are very young, the gills are divided by a thin layer of tissue within a major cavity (supplementary material) which, in photographs, may give a false impression that they are in one cavity and are able to fully retract. In fact, such an ontogenetic character was previously described (in Abraham, 1876) and it was the reason why Collingwood (1881) described the genus Albania.
It is well documented that in many species small juveniles are more likely to be camouflaged and/or found in hidden refuges than large adults. Nevertheless, how color ontogeny in marine invertebrates plays a role in juvenile protection is poorly understood (De Bruyn & Gosselin, 2014). For Hexabranchus spp. both hidden refuges and color ontogeny seem to be part of their survival strategy. Early juveniles of H. giganteus sp. nov., H. morsomus, H. sanguineus(except for the western Pacific lineage), and H. aureomarginatus were not found, supporting the hypothesis that during early development these species are hidden, perhaps in reef cavities or other habitats not easily accessible to divers. In contrast, H. lacer juveniles are common in shallow/tidal reefs and appear to migrate to deeper water when mature. The closely related species, H. sandwichensis, seems to have the same behavior, suggesting that this life strategy might be phylogenetically related.
Color variations can result from biological and physiological processes, but when a pattern exists (such as in Hexabranchus ontogeny), it is likely related to genetics (Bandaranayake, 2006), while the timing of ontogeny may be variable and influenced by changes in environmental factors (De Bruyn & Gosselin, 2014). Color and ontogeny can serve different purposes such as sexual attraction, protection, and predation. For nudibranchs, it is unlikely that color is related to sexual attraction since they do not have image-forming eyes. Nevertheless, fish can learn to recognize unpalatable color patterns and color may serve as camouflage or a warning signal (Aguado & Marin, 2007).
In all species of Hexabranchus, juveniles are lighter in overall color. The gain of pigmentation with maturity was also observed by Bruyn and Gosselin (2014) in three species of motile-shelled mollusks. Its functionality is unclear, but it may be a way to save energy at the early stages of development. Additionally, only a few pigments are commonly available at an embryonic stage and integumental pigments are usually synthesized with growth (and an increase in pigment synthesis rate) (Booth, 1990).
Color pattern embraces shapes, tonalities, colors, and brightness; a cryptic pattern represents a random sample of the background (Endler, 1978). It is reasonable to suspect that the gain of pigmentation in Hexabranchus spp. increases protection against predators; as with growth, they spend more time exposed to predators. Dorsal reddish and mottled patterns in animals resting in the open may act as disruptive coloration/camouflage under ambient light or even in the dark. On the other hand, with growth, the mantle of Hexabranchus species extends laterally, and distinctive colored patterns, often brighter than the dorsum, appear in this region. These outstanding patterns are only seen when animals unroll the mantle, usually due to a disturbance. Edmunds (1968) conducted a series of laboratory experiments concluding that the exposure of lateral pigmentation by Hexabranchus is probably a way to deceive predators rather than a warning mechanism because the studied animals only unrolled their mantle when prodded and at the time he did not detect skin glands. However, Hexabranchusspp. concentrate unpalatable macrolides in their dorsum (Pawlik et al., 1988), and therefore it could be that color in mature Hexabranchus plays primarily a camouflage role (rolled mantle) and a secondary/emergency alarming signal (unrolled) when physically disturbed.
Conclusion
Hexabranchus species have been fairly well studied and because of their large size, abundance and visibility have received much attention from scientists and nudibranch enthusiasts all over the world. Yet, until now their taxonomic status was highly debatable and controversial. This may be explained by their broad distributions, similar internal anatomy among different morphotypes, complex ontogenies, morphological changes due to behavior, several descriptions based on preserved specimens with no details of the living animals, failure of past studies to perform a full literature review and challenges in collecting/preserving/transporting large adult specimens (Fig. 27).
Map of species distributions. Full-filled colors represent specimens studied and colors with empty circles represent photograph records found in the literature and/or on-line. Yellow = Hexabranchus aureomarginatus. Pink = Hexabranchus giganteus sp. nov. Blue = Hexabranchus lacer. Brown = Hexabranchus morsomus. Red = Hexabranchus sanguineus. Green = Hexabranchus sandwichensis
This study represents the most complete phylogenetic review of the genus Hexabranchus to date, resurrecting three species and describing a new species. It also provides a complete review of synonyms, enormously facilitating further studies. Nevertheless, the question “How many species of Hexabranchus are there?” may not yet be fully solved. Some morphotypes could not be collected and the distinct morphotypes of H. sanguineus and H. lacer demand further attention. They are here regarded as the same species, but whether they are evolving as separate evolutionary units with restricted gene flow remains an open question, which may only be answered by a more robust genomic analysis (e.g., Hosegood et al., 2020).
The genus Hexabranchus shows some of the most extraordinary ontogenetic variations in nudibranchs. Color ontogeny is known to be linked to gene expression. Nevertheless, which genes are involved and what mechanism underlies color divergence in nudibranchs is virtually unknown. The complex ontogeny of Hexabranchus spp. offer a great opportunity for further genomic study to explore periodic patterns of development and color variations at the genomic level, a topic which is not yet well understood (Korshunova et al., 2021). Similarly, gill cavity ontogeny might be the key to understanding gill evolution in dorid nudibranchs (Martynov, 2011). The unique gill arrangements of the genus Hexabranchus may assist in this task. However, despite past attempts (Martynov, 2011), additional collection and careful morphological examination of juvenile Hexabranchus spp. are still needed to clarify whether this character is species or genus-specific and/or phylogenetically related.
Data availability
Data deposition information: TreeBASE. http://purl.org/phylo/treebase/phylows/study/TB2:S30027
References
-
Abraham, P. S. (1876). Notes on some genera of Nudibranchiate Mollusca, with notices of a new genus and of some hitherto undescribed species, in the collection of the British Museum. Annals and Magazine of Natural History including Zoology, Botany, and Geology, 18(4), 132–146.
-
Abraham, P. S. (1877). Revision of the Anthobranchiate Nudibranchiate Mollusca, with descriptions or notices of forty-one hitberto undescribed species (plates XXVII–XXX). Proceeding of Zoological Society of London, 1877: 196–269, pl. 28–30 [August].
-
Adams, A. (1848). Notes from a journal of research into the natural history of the countries visited during the voyage of H.M.S. Samarang, under the command of Captain Sir E. Belcher, C.B. London: 225–532. In: Edward Belcher. Narrative of the voyage of H.M.S. Samarang, during the years 1843–46; employed surveying the islands of the eastern archipelago; accompanied by a brief vocabulary of the principal languages, published under the authority of the Lords Commissioners of the Admiralty, vol. 2, 574 pp. Reeve, Benham & Reeve, London: 494
-
Adams, H., & Adams, A. (1858). The genera of recent mollusca arranged according to their organization. In three volumes. Plates (J. V. Voorst & P. Row (eds.)).
-
Aguado, F., & Marin, A. (2007). Warning coloration associated with nematocyst-based defences in aeolidiodean nudibranchs. Journal of Molluscan Studies, 73(1), 23–28. https://doi.org/10.1093/mollus/eyl026
-
Akaike, H. (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19(6), 716–723.
-
Alder, J., & Hancock, A. (1864). IV. Notice of a collection of Nudibranchiate Mollusca made in India by Walter Elliot, Esq., with Descriptions of several New Genera and Species. The Transactions of the Zoological Society of London, 5(3), 113–147.
-
Alfaro, M. E., Zoller, S., & Lutzoni, F. (2003). Bayes or bootstrap? A simulation study comparing the performance of Bayesian Markov chain Monte Carlo sampling and bootstrapping in assessing phylogenetic confidence. Molecular Biology and Evolution, 20(2), 255–266. https://doi.org/10.1093/molbev/msg028
-
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215(3), 403–410.
-
Apte, D., & Salahuddin, V. K. (2010). Record of Hexabranchus sanguineus (Ruppell & Leuckart, 1828) from Lakshadweep Archipelago, India. Journal of Bombay Natural History Society, 107(3), 261–262.
-
Atsushi, O. (1999). Opisthobranchs of Kerama Islands. Hankyu Communications Co., Ltd.
-
Atsushi, O. (2004). Opisthobranchs of Ryukyu Islands. Ruttles, inc. Tokyo, Japan.
-
Baba, K. (1936). Opisthobranchia of the Ryukyu (Okinawa) Islands. Journal Department Agriculture, Kyushu Imperial University, 5(1), 1–50, pls. 1–3.
-
Baba, K. (1988). Comment on Doris Lacera, from Timor. Indonesia. Shells and Sea Life,20(7), 11–12.
-
Bandaranayake, W. M. (2006). The nature and role of pigments of marine invertebrates. Natural Product Reports, 23, 223–255.
-
Basedow, H., & Hedley, C. (1905). South Australian nudibranchs, and an enumeration of the known Australian species. Transactions and Proceedings and Report of the Royal Society of South Australia, 29, 134–160, pls. I-XII.Bergh, L. S. R. (1877). Kritische utersuchung der Ehrenberg’schen Doriden. Jahrbücher Der Deutschen Malakozoologischen Gesellschaft, 4, 45–76.
-
Bergh, L. S. R. (1872). Malacologische Untersuchungen. In C. Semper (Ed.), Reisen im Archipel der Philippinen, Wissenschaftliche Resultate. Theil 1, Heft 3: 137–176, pls 17–20.
-
Bergh, L. S. R. (1877). Malacologische Untersuchungen. In Reisen im Archipel der Philippinen von Dr. Carl Gottfried Semper. Zweiter Theil. Wissenschaftliche Resultate. Band 2, Theil 2, Heft 12, pp. 495–546, pls. 58–61.
-
Bergh, L. S. R. (1878). Malacologische Untersuchungen. In Reisen im Archipel der Philippinen von Dr. Carl Gottfried Semper (pp. 547–601). Zweiter Theil.
-
Bergh, L. S. R. (1880). Malacologische Untersuchungen. In C. Semper (Ed.), Archipel der Philippinen (pp. 1–78). Zweirer Theil.
-
Bergh, L. S. R. (1891). Die cryptobranchiaten Dorididen. Zoologische Jahrbücher, Abtheilung für Systematik Geographie und Biologie der Thiere, 6(1), 103–144.
-
Bergh, L. S. R. (1892). Mit 20 Tafeln. Die Opisthobranchiata der Siboga-Expedition. Nudibranchiata. System der nudibranchiaten Gasteropoden. Malacol. Untersuchungen. III (Heft XVIII), p. 91–92, Pl. I, Fig. 1. Pl 5, Fig. 8.
-
Bergh, L. S. R. (1900). Ergebnisse einer Reise nach dem Pacific (Schauinsland 1896–1897). Die Opisthobranchier. Zoologische Jahrbücher, 13, 207–246.
-
Bergh, L. S. R. (1905). Die Opisthobranchiata. In Siboga Expeditie Report (Vol. 50).
-
Bertsch, H., & Johnson, S. (1982). Three new species of dorid Nudibranchs (Gastropoda: Opisthobranchia) from the Hawaiian Islands. The Veliger, 24(3), 208–221. http://www.biodiversitylibrary.org/item/134378
-
Bloch, M. E., & Schneider, J. G. (1801). M.E. Blochii, Systema Ichthyologiae iconibus cx illustratum. Post obitum auctoris opus inchoatum absolvit, correxit, interpolavit Jo. Gottlob Schneider, Saxo. Berolini. Sumtibus Auctoris Impressum et Bibliopolio Sanderiano Commissum. Pp i-lx + 1-584, Pls. 1–110.
-
Booth, C. L. (1990). Evolutionary significance of ontogenetic colour change in animals. Biological Journal of the Linnean Society, 40(2), 125–163. https://doi.org/10.1111/j.1095-8312.1990.tb01973.x
-
Briggs, J. C. J. C. (1999). Coincident biogeographic patterns: Indo-West Pacific Ocean. Evolution, 53(2), 326–335. https://doi.org/10.1111/j.1558-5646.1999.tb03769.x
-
Castresana, J. (2000). Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution, 17, 540–552.
-
Chavanich, S., Harris, L. G., & Viyakarn, V. (2010). Nudibranchs of Thailand. Biodiversity Research and Training Program (BRT).
-
Coleman, N. (1989). Nudibranchs of the South Pacific. Nevile Coleman’s Sea Australia Resource Center.
-
Coleman, N. (2001). 1001 Nudibranchs. Nevile Coleman’s Sea Australia Resource Center.
-
Coleman, N. (2008). Nudibranchs encyclopedia. Neville Coleman’s Underwater Geographic: Springwood.
-
Colgan, D. J., McLauchlan, A., Wilson, G. D. F., Livingston, S. P., Edgecombe, G. D., Macaranas, J., Cassis, G., & Gray, M. R. (1998). Histone H3 and U2 snRNA DNA sequences and arthropod molecular evolution. Australian Journal of Zoology, 46(5), 419. https://doi.org/10.1071/ZO98048
-
Colin, P. L., & Arneson, A. C. (1995). Tropical Pacific invertebrates: A field guide to the marine invertebrates occuring on tropical Pacific coral reefs, seagrass beds and mangroves. The Coral Reef Research Foundation, USA, 4, 296.
-
Collin, R., Diaz, M. C., Norenburg, J., Rocha, R. M., Sanchez, J. A., Schulze, A., Schwartz, M., & Valdés, A. (2005). Photographic identification guide to some common marine invertebrates of Bocas del Toro. Panama. Caribbean Journal of Science, 41(3), 638–707.
-
Collingwood, C. (1881). On some new species of nudibranchiate Mollusca from the eastern seas. Transactions of the Linnean Society, Zoology, Series 2, 2(2), 123–140, pls. 9–10.
-
Cuvier, G. L. C. F. D. (1804). Annales du Muséum d’histoire naturelle. 4: 453–465, 473, pl. 73, figs. 1–3b–c Aug. 1804.
-
Cuvier, G. L. C. F. D. (1817). Le Règne Animal, distribué d’après son organisation, pour servir de base à l’histoire naturelle des animaux et d’introduction à l’anatomie comparée, tome II, contenant les reptiles, les poissons, les mollusques et les annélides, xviii + 532 pp. Paris, Deterville.
-
Darriba, D., Taboada, G. L., Doallo, R., & Posada, D. (2012). jModelTest 2: More models, new heuristics and parallel computing. Nature Methods, 9, 772. https://doi.org/10.1038/nmeth.2109
-
De Bruyn, R. A. J., & Gosselin, L. A. (2014). Prevalence of ontogenetic changes in colour brightness among benthic invertebrates and their association with microhabitat shifts. Marine Ecology Progress Series, 498, 147–159. https://doi.org/10.3354/meps10626
-
Debelius, H. (1996). Nudibranchs and Sea Snails. Indo-Pacific Field Guide. From the Red Sea to South Africa. Across to the West Coast of the Americas. IKAN-Unterwasserarchiv.
-
Debelius, H., & Kuiter, R. H. (2007). Nudibranchs of the world. IKAN-Unterwasserarchiv.
-
Eales, N. B. (1953). A systematic and anatomical account of the opisthobranchia. The John Murray Expeditions 1933–34 Scientific Reports, 4(4), 91–98. https://www.biodiversitylibrary.org/item/195785
-
Edgar, R. C. (2004). MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32(4), 1792–1797. https://doi.org/10.1093/nar/gkh340
-
Edmunds, M. (1968). On the swimming and defensive response of Hexabranchus marginatus (Mollusca, Nudibranchia). Journal of the Linnean Society of London, 47(313), 425–429. https://doi.org/10.1111/j.1096-3642.1968.tb00550a.x
-
Edmunds, M. (1971). Opistobranchiate Mollusca from Tanzania (suborder: Doridacea). Zoological Journal of the Linnean Society, 59(4), 380–406. https://doi.org/10.1111/j.1096-3642.1971.tb00767.x/abstract
-
Eliot, C. N. E. (1903). Nudibranchiata, with some Own conclusion/opinion on its taxonomic status on the families and genera and description of a new genus, Doridomorpha. With plate XXXII. In: Gardiner, J. S. (1872–1946), The fauna and geography of the Maldive and Laccadive Archipelagoes: being the account of the work carried on and of the collections made by an expedition during the years 1899 and 1900, 2: 546–547.
-
Eliot, C. N. E. (1904a). On some nudibranchs from East Africa and Zanzibar. Part. IV. Proceedings of the Zoological Society of London, 1904, 380–406.
-
Eliot, C. N. E. (1904b). On some Nudibranchs from East Africa and Zanzibar. Part VI. Proceedings of the Zoological Society of London, 1(1), 1–10. https://doi.org/10.1111/j.1469-7998.1833.tb06418.x
-
Eliot, C. N. E. (1905). On some nudibranchs from the Pacific, including the genus Chromodoridella. Journal of Molluscan Studies, 5(1), 229-. https://doi.org/10.1093/oxfordjournals.mollus.a065930
-
Eliot, C. N. E. (1908). Reports on the marine biology of the Sudanese Red Sea – XI. Notes on a collection of nudibranchs from the Red Sea. Zoological Journal of the Linnean Society, 31(204), 86–122.
-
Ehrenberg, C. G. (1828–1831). Animalia evertebrata exclusis Insectis. Series prima. In: F. G. Hemprich & C. G. Ehrenberg, Symbolae physicae, seu icones et descriptiones Mammalium, Avium, Insectorum et animalia evertebra, quae ex itinere per Africam borealem et Asiam occidentalem studio nova aut illustrata redierunt. 126 pp. (1831), 10 pls (1828).
-
Endler, J. A. (1978). A predator’s view of animal color patterns. Evolutionary Biology, 319–364. https://doi.org/10.1007/978-1-4615-6956-5_5
-
Eydoux, J. F. T., & Souleyet, L. F. A. (1841). Voyage autour du monde Exécuté pendandt les annés 1836 et 1837 sur la Corvette La Bonite commandee par M. Vaillant Capitaine de Vaisseau. Atlas (A. Bertrand (ed.)). Ordre du Gouvernement.
-
Eydoux, J. F. T., & Souleyet, L. F. A. (1852). Voyage autour du monde exécuté pendant les années 1836 et 1837 sur la corvette La Bonite, commandée par M. Vaillant, captaine de vaisseau, publié par ordre du roi sous les auspices du Département de la marine, Paris, Zoologie Mollusques 2:1–664. [Nudibranchia pp. 393–459]
-
Folmer, O., Black, M., Hoeh, W., Lutz, R., & Vrijenhoek, R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3(5), 294–299. https://doi.org/10.1371/journal.pone.0013102
-
Francis, M. P. (1980). Habitat, food and reproductive activity of the nudibranch Hexabranchus sanguineus on Tongatapu Island. The Veliger, 22(3), 252–258.
-
Gohar, H. A. F., & Soliman, G. N. (1963). The biology and development of Hexabranchus sanguineus (Rüp and Leuck.). Marine Biological Station, Al-Ghardaga, Egypt, 12, 219–247, pls. 1–3.
-
Gosliner, T. M. (1987). Nudibranchs of southern Africa: A guide to opisthobranch molluscs of southern Africa. Sea Challengers. South Africa.
-
Gosliner, T. M., Behrens, D. W., & Valdés, Á. (2008). Indo-Pacific nudibranchs and sea slugs: A field guide to the world’s most diverse fauna. Sea Challengers Natural History Books.
-
Gould, A. A. (1852). United States Exploring Expedition. During the years 1838, 1839, 1840, 1841, 1842. Under the command of Charles Wilkes, U.S.N. Vol. XII.
-
Gray, J. E. (1847). A list of the genera of recent Mollusca, their synonyma and types. Proceedings of the Zoological Society of London, 15, 129–219.
-
Gray, J. E. (1850). In: Gray, M. E., Figures of molluscous animals, selected from various authors. Longman, Brown, Green and Longmans, London. Vol. 4, iv + 219 pp. (August) [Frontispiece (portrait of Mrs. Gray); pp. ii–iv (preface); 1–62 (explanation of plates 1–312 in Volumes 1–3); pp. 63–124 (systematic arrangement of figures); 127–219 (reprint of Gray 1847).
-
Gutiérrez, M. C., Ortea, J., Rivero, N., Tucker, G. C., Malaquias, M. A. E., & Narciso, S. (2015). The opisthobranch gastropods (Mollusca: Heterobranchia) from Venezuela: An annotated and illustrated inventory of species. Zootaxa, 4034(2), 201–256. https://doi.org/10.11646/zootaxa.4034.2.1
-
Haas, F. (1920). Opisthobranchier aus verschiedenen warmen Meeren. Archiv Für Molluskenkunde, 52, 138–142.
-
Habe, T. (1961). Coloured illustrations of the shells of Japan (II). Hoikusha, Osaka. xii + 183 + 42 pp., 66 pls. page(s): 92, and appendix p. 33.
-
Hägg, R. (1901). Two new opisthobranchiathe Mollusca from the Red Sea. Accompanied by a list of references to the genus Notarchus Cuvier and Hexabranchus Ehrenbergh. In: Results of the Swedish Zoological Expeditions to Egypt and the White Nile 1901 under direction of L. A. Jägerskoiöld, 6: 5–7, pl. 1, figs. 4–5.
-
Hallas, J. M., Chichvarkhin, A., & Gosliner, T. M. (2017). Aligning evidence: Concerns regarding multiple sequence alignments in estimating the phylogeny of the nudibranchia suborder doridina. Royal Society Open Science, 4(10). https://doi.org/10.1098/rsos.171095
-
Hervé, J.-F. (2010). Guide des nudibranches de Nouvelle-Calédonie et autres Opistobranches. Catherine Ledru.
-
Hoover, C. A., Padula, V., Schrödl, M., Hooker, Y., & Valdés, Á. (2017). Integrative taxonomy of the Felimare californiensis and F. ghiselini species complex (Nudibranchia: Chromodorididae), with description of a new species from Peru. Journal of Molluscan Studies, 1855(September), 1–15. https://doi.org/10.1093/mollus/eyx035
-
Hosegood, J., Humble, E., Ogden, R., de Bruyn, M., Creer, S., Stevens, G. M. W., Abudaya, M., Bassos-Hull, K., Bonfil, R., Fernando, D., Foote, A. D., Hipperson, H., Jabado, R. W., Kaden, J., Moazzam, M., Peel, L. R., Pollett, S., Ponzo, A., Poortvliet, M., … Carvalho, G. (2020). Phylogenomics and species delimitation for effective conservation of manta and devil rays. Molecular Ecology, 29(24), 4783–4796. https://doi.org/10.1111/mec.15683
-
Huelsenbeck, J. P., & Rannala, B. (2004). Frequentist properties of bayesian posterior probabilities of phylogenetic trees under simple and complex substitution models. Systematic Biology, 53(6), 904–913. https://doi.org/10.1080/10635150490522629
-
International Commission on Zoological Nomenclature. (2000). The International Code of Zoological Nomenclature. https://www.iczn.org/the-code/the-code-online/
-
Kay, E. A., & Young, D. K. (1969). The Doridacea (Opisthobranchia; Mollusca) of the Hawaiian Islands. Pacific Science, 23(2), 172–231.
-
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., Duran, C., & Thierer, T. (2012). Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28(12), 1647–1649.
-
Kelaart, E. F. (1858). Description of new and little-known species of Ceylon Nudibranchiate Molluscs, and Zoophytes. Journal of the Royal Asiatic Society Ceylon, Colombo, 3(1), 91–93.
-
Kekkonen, M., Mutanen, M., Kaila, L., Nieminen, M., & Hebert, P. D. N. (2015). Delineating species with DNA barcodes: A case of Taxon dependent method performance in moths. PLoS ONE, 10(4). https://doi.org/10.1371/journal.pone.0122481
-
King, D. & Fraser, V. (2014). The Reef Guide. Fishes, corals, nudibranchs & other invertebrates. East & South Coasts of Southern Africa. Struik Nature.
-
Korshunova, T. A., Driessen, F. M. F., Picton, B. E., & Martynov, A. V. (2021). The multilevel organismal diversity approach deciphers difficult to distinguish nudibranch species complex. Scientific Reports, 11(183223), 1–22. https://doi.org/10.1038/s41598-021-94863-5
-
Korshunova, T., Fletcher, K., Picton, B., Lundin, K., Kashio, S. H. O., Sanamyan, N., Sanamyan, K., Padula, V., Schrödl, M., & Martynov, A. (2020). The Emperor’s Cadlina, hidden diversity and gill cavity evolution: New insights for the taxonomy and phylogeny of dorid nudibranchs (Mollusca: Gastropoda). Zoological Journal of the Linnean Society, 1–66.
-
Korshunova, T., Martynov, A., & Picton, B. (2017). Ontogeny as an important part of integrative taxonomy in Tergipedid aeolidaceans (Gastropoda: Nudibranchia) with a description of a new genus and species from the Barents Sea. Zootaxa, 4324(1), 1–22. https://doi.org/10.11646/zootaxa.4324.1.1
-
Kumar, S., Stecher, G., & Tamura, K. (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33(7), 1870–1874. https://doi.org/10.1093/molbev/msw054
-
Leigh, E. G., O’Dea, A., & Vermeij, G. J. (2014). Historical biogeography of the Isthmus of Panama. Biological Reviews, 89(1), 148–172. https://doi.org/10.1111/brv.12048
-
Mahmoud, M. A. M., & Raafat, M. (2016). Spawn and early larval development of Spanish dancer nudibranch Hexabranchus sanguineus (Rüppell and Leuckart, 1828) (Gastropoda: Nudibranchia). Journal of Fisheries and Aquatic Science, 3, 1–8. https://doi.org/10.3923/jfas.2016
-
Marcus, E., & Marcus, E. (1968). Opisthobranchs from Curaçao and faunistically related regions. Studies on the Fauna of Curaçao and Other Caribbean Islands, 122, 1–129.
-
Marcus, Ev., & Marcus, Er. (1962). Opisthobranchs from Florida and Virgin Islands. Bulletin of Marine Science of the Gulf and Caribbean, 12(3), 450–488.
-
Marshall, J. G., & Willan, R. C. (1999). Nudibranchs of Heron Island, Great Barrier Reef. A survey of the Opisthobranchia (Sea Slugs) of Heron and Wistari Reefs. Backhuys Publishers Leiden.
-
Martín-Hervás, M. del R., Carmona, L., Malaquias, M. A. E., Krug, P. J., Gosliner, T. M., & Cervera, J. L. (2021). A molecular phylogeny of Thuridilla Bergh, 1872 sea slugs (Gastropoda, Sacoglossa) reveals a case of flamboyant and cryptic radiation in the marine realm. Cladistics, 1–30. https://doi.org/10.1111/cla.12465
-
Martynov, A. V. (2011). From ‘tree-thinking’ to ‘cycle-thinking’: Ontogenetic systematics of nudibranch molluscs. Thalassas, 27(2), 193–224.
-
Mcdonald, G. (2021). Nudibranch systematic index compiled by Gary McDonald.
-
McDonald, G. R., & Nybakken, J. (1997). A list of the worldwide food habits of nudibranchs. … of California, Santa Cruz Web Site— …, 1–331. https://doi.org/10.1016/j.ympev.2011.04.003
-
MolluscaBase eds. (2023a). MolluscaBase. Caribranchus Ortea, Caballer & Moro, 2003. Accessed through: World Register of Marine Species at: https://www.marinespecies.org/aphia.php?p=taxdetails&id=758609 on 2023a–03–09
-
MolluscaBase eds. (2023b). Hexabranchus sanguineus (Rüppell & Leuckart, 1830). World Register of Marine Species. MolluscaBase. https://www.marinespecies.org/aphia.php?p=taxdetails&id=220381
-
MolluscaBase eds. (2023c). MolluscaBase. Doris sandwichensis (Gray, 1850). Accessed through: World Register of Marine Species. Retrieved March 13, 2023, from https://www.marinespecies.org/aphia.php?p=taxdetails&id=549815
-
Mörch, O. A. L. (1863). Contributions la faune malacologique des Antilles danoises. Journal De Conchyliologie, 11, 21–43.
-
Morton, J. E. (1964). Locomotion. In K. M. Wilbur & C. M. Yonge (Eds.), Physiology of Molluscs (Vol. 1, pp. 383–433). Academic Press. https://doi.org/10.1038/204818a0
-
Nakano, R. (2004). Opisthobranchs of Japan Islands. TBS-Brittanica Co.
-
Nakano, R. (2018). Field guide to sea slugs and nudibranchs of Japan. Bun-ichi Sogo Shuppan Co.
-
Neuhaus, J., Rauch, C., Bakken, T., Picton, B., Pola, M., & Malaquias, M. A. E. (2021). The genus Jorunna (Nudibranchia: Discodorididae) in Europe: A new species and a possible case of incipient speciation. Journal of Molluscan Studies, 87(4), eyab028. https://doi.org/10.1093/mollus/eyab028
-
Nimbs, M. J., & Smith, S. D. A. (2016). An illustrated inventory of the sea slugs of New South Wales, Australia (Gastropoda: Heterobranchia). The Royal Society of Victoria, 128, 44–113. https://doi.org/10.1071/rs16011
-
Odhner, N. H. (1934). The nudibranchiata of British antarctic expedition. British antarctic (“Terra Nova”) expedition, 1910. Natural history reports. Zoology, 7, 229–310.
-
Ortea, J., & Buske, Y. (2018). Lista inicial ilustrada de las babosas marinas (Heterobranquios) de la expedición Madibenthos, realizada en 2016 en Martinica (Antillas Menores, mar Caribe). Revista De La Academia Canaria De Ciencias, 30, 67–102.
-
Ortea, J., Caballer, M., & Moro, L. (2002). Descripción de los individuos jovenes de Hexabranchus mormosus Marcus & Marcus, 1962 (Mollusca, Nudibranchia) con algunas consideraciones sobre la sistemática de la familia Hexabranchidae. Revista de La Academia Canaria de Ciencias, 3–4, 193–200. http://www.researchgate.net/profile/J-Fernando_Pascual-Sanchez/publication/259383232_Resolucin_general_del_problema_inverso_del_Clculo_de_Variaciones_el_teorema_de_Tonti_(1984)/links/0deec52b479ad4e84e000000.pdf
-
Ortea, J., Espinosa, J., Caballer, M., & Buske, Y. (2012). Initial inventory of de sea slugs (Opisthobranchia and Sacoglossa) from the expedition Karubenthos, held in May 2012 in Guadeloupe (Lesser Antilles, Caribbean Sea). Revista De La Academia Canaria De Ciencias,24, 153–182.
-
Ortea, J. A., Caballer M., & Moro, L. (2003) [for 2002]. Descripción de los individuos jovenes de Hexabranchus mormosus Marcus & Marcus, 1962 (Mollusca, Nudibranchia) con algunas consideraciones sobre la sistemática de la familia Hexabranchiadae. Revista de la Academia Canaria de Ciencias, 14(3–4), 193–200.
-
Ostergaard, J. M. (1955). Some opisthobranchiate Mollusca from Hawaii. Pacific Science,9(2), 110–136. https://doi.org/10.2307/2752573
-
Palumbi, S. R., Martin, A., Romano, S., McMillan, W. O., Stice, L., & Grabowski, G. (2002). The simple fool’s guide to PCR version 2. University of Hawaii, 96822(808), 1–45. https://doi.org/10.1186/s13620-015-0060-3
-
Pawlik, J. R., Kernan, M. R., Molinski, T. F., Harper, M. K., & Faulkner, J. D. (1988). Defensive chemicals of the Spanish dancer nudibranch Hexabranchus sanguineus and its egg ribbons: Macrolides derived from a sponge diet. Journal of Experimental Marine Biology and Ecology, 119, 99–109.
-
Pease, W. H. (1860). Descriptions of new species of Mollusca from the Sandwich Islands, Part I. Proceedings of the Zoological Society of London, 28(18–36), 141–148.
-
Pittman, C. (2011). Speculation on the taxonomy of Hexabranchus. Sea Slugs of Hawai’i. http://seaslugsofHawaii.com/general/Hexabranchus-article.html
-
Pruvot-Fol, A. (1934). Archives du Muséum national d’histoire naturelle, series 6 (Vol. 11). Ed. du Muséum. https://www.biodiversitylibrary.org/item/280451
-
Quoy, J. R. C., & Gaimard, J. P. (1832). Voyage de découvertes de l’Astrolabe exécuté par ordre du Roi, pendant les annees 1826–1827-1828-1829, sous le commandement de M. J. Dumont d’Urville. Zoologie, Mollusca, 2, 1–320.
-
Ribes-Beaudemoulin, S. R., Trentin, F., Bidgrain, P., Huet, R., Diringer, A., & Cadet, C. (2019). Coquillages nudibranches & autres mollusques marins (Biodiversi). Éditions du Cyclone.
-
Risbec, J. (1928). Contribution a l’etude des nudibranches Néo-Calédoniens. Thèse présentée à la faculté des sciences de l’Université de Paris pour obtenir le grade de docteur ès-sciences naturelles. Faune Des Colonies Française, 2(1), 1–328, pls. 1–12.
-
Ronquist, F., & Huelsenbeck, J. P. P. (2003). MRBAYES 3, Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572–1574.
-
Rüppel, E., & Leuckart, F. S. (1828). Neue wirbellose Thiere des Rothen Meeres. Atlas Zu Der Reise Im Nordlichen Afrika von Eduard Ruppell, 1–22.
-
Rüppell, E., & Leuckart, F. S. (1830). Mollusk Atlas zu der Reise im nördlichen Afrika von Eduard Rüppell: 28–29, pl. 8, fig. 1.
-
Rüppell, E., & Leuckart, F. S. (1828–1830). Mollusca. In: Atlas zu der Reise im nordlichen Afrika von Eduard Rüppell. 1. Abth. Zoologie. 5. Neue wirbellose Thiere des Rothen Meeres. Frankfurt, H.L. Brönner. pp. 15–22, pls. 1–4 [1828]; pp. 23–47, pls. 7–11 [1830]
-
Russell, H. D. (1971). Index Nudibranchia a catalog of the literature 1554–1965, iv + 141 pp. Delaware Museum Natural History.Salvat, B. & Bacchet, P. (2011). Guide des Recifs Coralliens de Tahiti et Ses Iles. Collecione Naturale et Environement O’Océanie.
-
Salvat, B., & Bacchet, P. (2011). Guide des récifs coralliens de Tahiti et ses îles. Au vent des îles éd..
-
Saville-Kent, W. S. (1897). The Naturalist in Australia. London: Chapman & Hall, 150-151, pl. 5.
-
Schauinsland, H. H. (1899). Three months on a coral island (Laysan). Atoll Research Bulletin, 426, 1–674. papers2://publication/uuid/BEA3F4A8-A770–4CE8-BAE3-E04149760516
-
Sherborn, C. D. (1901). Dates of publication of the zoological and botanical portions of some French voyages: part II (Vol. 8).South-west Indian Ocean Seaslug site. (2011). South-west Indian Ocean Seaslug site. Dendrodoris Sp. 1. http://seaslugs.free.fr/nudibranche/a_intro.htm
-
Stöver, B. C., & Müller, K. F. (2010). TreeGraph 2: Combining and visualizing evidence from different phylogenetic analyses. BMC Bioinformatics, 11(7), 1–9. https://doi.org/10.1186/1471-2105-11-7
-
Stromvoll, J., & Jones, G. (2019). A guide to the sea slugs of Maputoland Coast. Castle Graphics Northe (Pty) Ltd.
-
Thompson, T. E. (1972). Observations on Hexabranchus from the Australian Great Barrier Reef (Gastropoda: Opisthobranchia). The Veliger, 15, 1–5.
-
Tibiriçá, Y., Pola, M., & Cervera, J. L. (2017). Astonishing diversity revealed: An annotated and illustrated inventory of Nudipleura (Gastropoda: Heterobranchia) from Mozambique.Zootaxa, 4359(Monograph), 1–133. https://doi.org/10.11646/zootaxa.4359.1.1
-
Tonozuka, T. (2003). Opisthobranchs of Bali and Indonesia. Hankyu Communications Co.
-
Valdés, À. (2002). How many species of Hexabranchus (Opisthobranchia: Dorididae) are there? Mollusca Research, 22, 289–301.
-
Valdés, Á., & Fahey, S. J. (2006). Dorid nudibranchs described by J.E. Gray in M.E. Gray, 1842–1857 (Mollusca: Opisthobranchia). Records of the Western Australian Museum Supplement 69:95–102.
-
Valdés, Á., Hamann, J., Behrens, D. W., & Dupont, A. (2006). Caribbean sea slugs. Sea Challengers Natural History Books.
-
Vayssière, A. (1912). Recherches zoologiques et anatomiques sur les Opisthobranches de la mer Rouge et du golfe d’Aden. Opisthobranches (suit et fin), Marseniadés, Oncidiidés. Journal de Conchyliologie, 14(246–247).
-
Yonow, N. (2001). Results of the Rumphius Biohistorical Expedition to Ambon (1990): Part 11. Doridacea of the families Chromodorididae and Hexabranchidae (Mollusca, Gastropoda, Opisthobranchia, Nudibranchia), including additional Moluccan material. Zoologische Mededelingen, 75(1), 1–50.
-
Yonow, N. (2008). Sea slugs of the Red Sea. Pensoft.
-
Zamora-Silva, A., & Malaquias, M. A. E. (2018) [nomenclatural availability: 2017]. Molecular phylogeny of the Aglajidae head-shield sea slugs (Heterobranchia: Cephalaspidea): New evolutionary lineages revealed and proposal of a new classification. Zoological Journal of the Linnean Society, 183(1), 1–51. https://doi.org/10.1093/zoolinnean/zlx064
-
Zhang, J., Kapli, P., Pavlidis, P., & Stamatakis, A. (2013). A general species delimitation method with applications to phylogenetic placements. Bioinformatics, 29(22), 2869–2876. https://doi.org/10.1093/bioinformatics/btt499
-
Zhou, Z., & Mittal, R. (2017). Swimming without a spine: Computational modeling and analysis of the swimming hydrodynamics of the Spanish Dancer. Bioinspiration & Biomimetics, 13(1), 015001. https://doi.org/10.1088/1748-3190/aa9392
Acknowledgements
We thank the Florida Museum, the Zoological Museum of Bergen University, the Museu Nacional de História Natural e da Ciência, the Universidade de Lisboa, the Museu de História Natural de Maputo, and the Victoria Museum who lent us specimens. We are grateful to Isabel da Silva from the Universidade do Lúrio, Amanda M. Bemis and Terry A. Lott from the Florida Museum of Natural History, and Melaine Mackenzie from the Museum Victoria for organizing and sending us samples. We thank Jon Langdon Wright and Jenny Strömvoll (Back to Basics Adventures) for their valuable help collecting specimens in Mozambique. Equally, we are grateful to Gary Cobb for his help in collecting specimens in Australia. We are also grateful to have access to the CIPRES Science Gateway to carry out phylogenetic analyses and the Biodiversity Heritage Library for making available the original descriptions revised here. We would like to highlight the importance of all underwater photographers and citizen scientists in helping solve complex cases as this one and we are particularly grateful for those who contributed with photos for this project: Laurent Beche, Laura Blackshaw, Stewart Clark,Gary Cobb, Steven Daniel, Pearlfisher Dietmar, Alex Dutcher, John Earle, Pauline Fiene, David Fleetham, Keiko French, Michael Ghiselin, Reindert Grooters & Mieke Snoek, John Hoover, Sean Dae Houlihan, Christovan Jarsveld, Christopher Cadet, Daniel, Jennings-Kam, Scott Johnson, Georgina Jones, Irma Korb, Jenny Strömvoll, Danila Mansfield (blog “Notdunroamin”), Fabian Michenet, Geir Næss, Gustav Paulay, Chantal Perrin, Daniel Petitmermet, Miguel Ramirez, Anthony Renaud, Ern Rod, David Rolla, Dan Schofield, Mieke Snoek & Reindert Grooters, Sara Thiebaud, Erwan Thollon, Philip Thomas, Ángel Valdés, Karolle Wall, Debra Woolley, and Dov Zingerman, Celina Azevedo, Philippe Bacchet, Edward Blackshaw, Melodie Caussat, Mickey Charteris, Nat Evans, Jessica Goodheart, Carole Harris, Linda Ianniello, Sven Kahlbrock, Amanda Johnston, Eric Kaye, Roman Maral, Linda Marie, Gerald McCormack, Kirby Morejohn, David Parker, Mark Patterson. We are also very grateful to the anonymous reviewer who helped us improve our manuscript, as well as to Philippe Bouchet and Serge Gofas for their valuable comments. We thank the reviewers and the editor for their valued time and comments.
Funding
Funding for open access publishing: Universidad de Cádiz/CBUA. This project was partially funded by funds received by the research group “Marine Biology and Fisheries’ PAIDI RNM-213.”
Ethics declarations
Ethics approval
No approval of research ethics committees was required to accomplish the goals of this study because the work was conducted with an unregulated invertebrate species.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tibiriçá, Y., Pola, M., Pittman, C. et al. A Spanish dancer? No! A troupe of dancers: a review of the family Hexabranchidae Bergh, 1891 (Gastropoda, Heterobranchia, Nudibranchia). Org Divers Evol (2023). https://doi.org/10.1007/s13127-023-00611-0
- Received
- Accepted
- Published
- DOIhttps://doi.org/10.1007/s13127-023-00611-0
Keywords
- Hexabranchus
- Integrative taxonomy
- Nudibranchs
- Ontogeny
- Phylogeny
- Species delimitation